First Successful Use of Low Dose Amoxicillin-Clavulanic Acid in Management of Drug Resistant Tuberculosis
Gyanshankar Mishra1, Jose A Caminero2
1 Associate Professor, Respiratory Medicine, Indira Gandhi Government Medical College, Nagpur, Maharashtra, India.
2 Consultant, Pneumology Department, General Hospital of Gran Canaria “DR. Negrin”, Las Palmas GC Spain Las Palmas, Gran Canaria, Spain.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Gyanshankar Mishra, Department of Respiratory Medicine, Indira Gandhi Government Medical College, CA Road, Nagpur-440018, Maharashtra, India.
E-mail: gpmishra81@gmail.com
Resistance to anti-tuberculosis drugs is a formidable obstacle to effective tuberculosis (TB) care. A case of Pre-XDR (Pre-Extensive Drug Resistant) pulmonary TB was reported with limited therapeutic options, where low dose amoxicillin-clavulanic acid was used as an integral component of a successful regime for the first time in medical literature. World health organisation recommends giving amoxicillin-clavulanate along with meropenem as one of the therapeutic options in drug resistant tuberculosis, where clavulanate and not amoxicillin is being relied upon for anti TB activity. However, across the spectrum of dosage of amoxicillin-clavulanate combination, the dose of clavulanate is constant at 125 mg, whereas the dose of amoxicillin varies at 250 mg, 500 mg and 875 mg. This explains the rationale behind the use of low dose amoxicillin-clavulanate in combination with meropenem as a part of treatment regime for drug resistant TB.
Amoxicillin-clavulanate, Low dose anti-TB drugs, Meropenem, Tuberculosis
Case Report
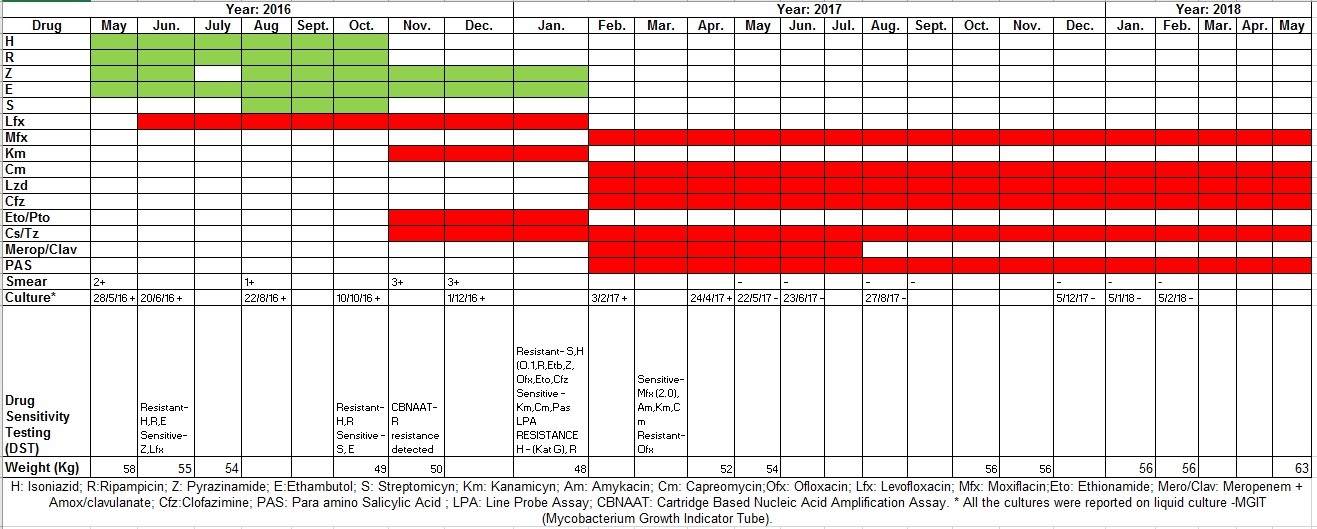
A 22-year-old male presented to the outdoor patient department in November 2016 with complaints of cough with expectoration associated with occasional streaky haemoptysis and low-grade evening rise in fever for last eight months. The patient had history of anorexia and weight loss of 8 kg (58 kg to 50 kg) in last six months. The patient was a case of sputum smear positive pulmonary TB since last six months and was diagnosed as a case of drug resistant pulmonary TB (Multi drug resistant (MDR) TB – Resistant to INH and rifampicin) five months prior, but was still on first line anti-TB drugs at the time of current clinical presentation. The detailed anti tuberculosis therapy drug history along with serial sputum smear and culture reports has been depicted in [Table/Fig-1] (Drugogram). General examination revealed no significant finding. There was no history of other co-morbidities like Diabetes and HIV (Human Immunodeficiency Virus) infection. There was no history of TB contact in the family or neighbourhood. Respiratory system examination revealed decreased breath sounds in right infraclavicular, infra-axillary and supraclavicular area.
Investigations revealed the following: sputum AFB (Acid Fast Bacilli) smear was positive with 2 + grading & sputum CBNAAT (Cartridge Based Nucleic Acid Amplification Test) detected Mycobacterium tuberculosis with rifampicin resistance. Chest radiograph was suggestive of right upper zone cavity with consolidation with left mid zone infiltrates. For rifampicin resistant TB, a regime for MDR TB was initiated under PMDT (Programmatic Management of Drug Resistant Tuberculosis) with pre-treatment evaluation as per programmatic protocol. Pre-treatment evaluation included the following investigations: Complete blood count with haemoglobin, liver function tests, renal function tests (Blood urea and serum creatinin), random blood sugar, thyroid function tests (T3, T4 and TSH) and urine routine and microscopy examination. These investigations were within normal limits. Serological test for HIV infection by ELISA (Enzyme-Linked Immunosorbent Assay) method was negative. Baseline psychiatric evaluation was normal.
As per patient’s weight (50 kg), patient was initiated on the following regime (day one)- Inj kanamycin 750 mg im OD, Tb. levofloxacin 1 gm od, Tb. ethambutol 1200 mg od, Tb. ethionamide 750 mg od, Tb. pyrazinamide 1500 mg od, Cp. cycloserine 750 mg od alongwith Tb pyridoxine 100 mg od. Also, patient’s sputum sample for first and second line culture DST (Drug sensitivity testing) was sent. Finally, by 9/1/2017 the following sputum reports were available: 1) LPA (Line Probe Assay) detected Rifampicin resistance & INH (Isonicotinylhydrazide or Isoniazid) resistance by Kat G mutation; 2) MGIT (Mycobacteria Growth Indicator Tube) 320 DST detected resistance to streptomycin, INH (0.1), rifampicin, ethambutol, pyrazinamide, ofloxacin, ethionamide, clofazimine and sensitivity to kanamycin, capreomycin and PAS (Para Amino Salicylic acid).
Based on clinical non-response to the regime and availability of updated drug and sensitivity results, a diagnosis of pre-XDR TB with fluoroquinolone resistance was made. The patient’s regime for drug resistant TB was revised on day eighty as follows (weight: 48 kg): meropenem 1 gm iv tid + amoxicillin-clavulanic acid (250 mg/125 mg) 375 mg tid, Inj. capreomycin 1 gm im od, moxifloxacin 400 mg od, linezolid 600 mg od, clofazimine 200 mg OD, cycloserine 750 mg od and PAS granules 12 gm OD along with pyridoxine 100 mg od. The rationale for drug selection along with their consideration as core drugs in the regime is mentioned in [Table/Fig-2].
Rationale for drug selection in the regime administered to the patient.
| Sr. No. | Name of the Drug | Whether considered as core drug – Yes/No | Reason |
|---|
| 1 | Moxifloxacin | No. | Moxifloxacin DST was not available at start of the treatment regime and DST had shown resistant to Fluoroquinolone class drug (ofloxacin). On 7/3/17 the DST of moxifloxacin 2.0 (high dose) was reported to be sensitive, after which the dose of moxifloxacin was changed from 400 mg od to 800 mg od. At that time, it could be considered as a core drug in the regime. |
| 2 | Capreomycin | Yes | DST showed sensitivity to capreomycin and patient had not received capreomycin in the past. Therefore, Capreomycin could be considered as a core drug in the regimen. |
| 3 | Cycloserine | No | The DST to this drug was not available and the patient had received this drug for more than one month as a part of the regime to which the patient did not respond clinically. Therefore, cycloserine could not be accounted among the possible effective drugs of the regimen. |
| 4 | Linezolid | Yes | DST report was not available for this drug but the patient had not received this drug previously. Therefore, linezolid could be considered as a core drug in the regimen. |
| 5 | Clofazimine | No | DST was resistant to this drug but this drug was included in the regime since the patient had not received this drug previously and also the DST to clofazimine is not reliable. |
| 6 | Meropenem, Amoxicillin- Clavulanic Acid | Yes. | The patient had no history of exposure to this drug combination previously for a period of more than one month. The combination of meropenem + clavulanate is considered as a single drug, but could be accounted as one of the possible effective drugs in the regimen. |
| 7 | PAS | No | DST showed sensitivity to this drug even though the patient had received this drug as a part of previous regime for MDR TB. Therefore, PAS could not be accounted among the possible effective drugs of the regimen. |
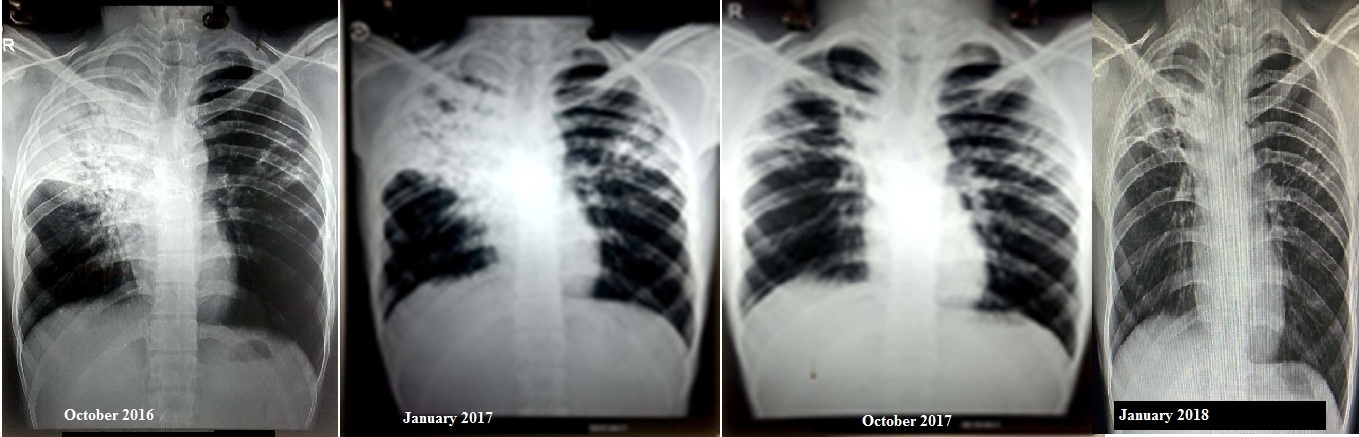
The above regime was continued for a period of six months following which meropenem-amoxicillin-clavulanate was stopped. Injection capreomycin was stopped after a period of nine months. The rest of the drugs were continued and are ongoing. At present, the patient has completed twelve months of treatment along with clinico-radiological improvement and sputum culture conversion. There has been a weight gain of 8 kg by the patient while on above regime. The serial chest radiographs in months of October 2016, January 2017, October 2017 and January 2018 respectively showing radiological improvement are shown in [Table/Fig-3].
Serial chest radiographs showing radiological improvement.
Discussion
Drug resistant tuberculosis is a man-made phenomenon [1]. The careful perusal of patient’s initial clinical records revealed the following potential factors for drug resistance in the patient’s therapy that could have been avoided – Co-prescription of fluoroquinolone in suboptimal dosing with first line drugs initially, suboptimal dose of pyrazinamide in split dosing for a brief period and continuation of first line drugs for five months after initial diagnosis of drug resistant TB before being referred to us [2,3]. Also, baseline sputum CBNAAT or LPA was highly desirable in the current patient and would have picked up any primary drug resistance if present at the beginning [4].
Once a modified regime for patient’s drug resistant TB was initiated on 23/1/2017, the patient showed good clinico-radiological improvement with successful sputum culture conversion. One of the highlights of the current regime was use of low dose of amoxicillin-clavulanate in the treatment of adult drug resistant TB. World health organisation recommends giving amoxicillin-clavulanate along with meropenem (or imipenem) as one of the therapeutic options in drug resistant tuberculosis [5]. The rationale behind this combination is that betalactam like meropenem (or other carbapenem like imipenem or ertapenem) has potential antimycobacterial activity whereas clavulanate, a beta-lactamase inhibitor irreversibly inactivates mycobacterial beta-lactamase, Blac thereby protecting meropenem from the beta-lactamase activity of Blac [6]. Currently absence of any single combination of meropenem and clavulanate necessitates both the drugs to be given separately. The reason cited for recommending the amoxicillin–clavulanate combination being that clavulanate is only available in formulations with amoxicillin. Thus, amoxicillin is not being relied upon for any anti-tuberculosis action in the recommended combination. The dose of amoxicillin-clavulanate currently used in drug resistant TB is 500 mg/125 mg three times daily [6]. The Indian guidelines recommend the dose of amoxicillin-clavulanate, (875mg/125mg) as one tablet bid for patients weighing between 16 to 45 kg, further increased to two tablets in morning and one in evening for patients weighing more than 45 kg [7]. However, the absence of option of carbapenem in the recent Indian PMDT guidelines requires to be urgently addressed as amoxicillin-clavulanate, is currently recommended by the WHO to be/ administered in combination with a carbapenem (like meropenem/imipenem/ertapenem) only [5,8].
Currently amoxicillin-clavulanate combined pill is available in 250 mg/125 mg, 500 mg/125 mg and 875 mg/125 mg strengths [9]. Thus, across the spectrum of dosage of amoxicillin-clavulanate combination, the dose of clavulanate is constant at 125 mg, whereas the dose of amoxicillin varies at 250 mg, 500 mg and 875 mg. Hence, it would be rational to prescribe the combination with minimum dosage of amoxicillin i.e., amoxicillin/clavulanate (250mg/125 mg). This would ensure that the dose of amoxicillin, which is not being administered for any anti-tuberculosis activity, is kept at minimum thereby potentially minimising the side effects and drug interactions related to amoxicillin, especially considering the long duration for which it needs to be administered [10].
Management of drug resistant TB (Tuberculosis) is a delicate balance of efficacy and toxicity of the therapeutic options limited by the disease condition itself. Judicious use of the available drugs is pertinent to the success of any drug resistant TB regime. The above patient was a case of pre-XDR pulmonary TB with limited therapeutic options where low dose amoxicillin-clavulanic acid has been used an integral component of a successful regime for the first time in medical literature. The difficulty in management of these patients can be gauged by a humble treatment success rate of 38.9% reported previously in patients with Pre-XDR TB with fluoroquinolone resistance [11].
To the best of our knowledge, the current case is the first such reported case of drug resistant TB in medical literature where low dose amoxicillin-clavulanate has been used in combination with meropenem as an important component of a successful regime. The clinico-radiological improvement along with successful culture conversion during the twelve months ongoing regime points towards an increased potential role of low dose amoxicillin-clavulanic acid in the treatment regime of such drug resistant patients. However, amoxicillin-clavulanic acid is also a very commonly used antibiotic in the management of other respiratory infections in the community. This aspect needs to be addressed and empirical use of amoxicillin-clavulanic acid should be discouraged in treating respiratory infections. The recent Indian guidelines on management of TB has already recommended against empirical use of amoxicillin-clavulanic acid in treating patients who are presumptive TB cases which is an excellent recommendation in today’s scenario of drug resistant TB considering the future potential of this combination [7].
Conclusion
The present case highlights the need of further research on low dose amoxicillin-clavulanate combination with carbapenems, paving way for its increased role in treatment of drug resistant TB in the near future.
[1]. Frieden TR, Toman’s tuberculosis: case detection, treatment and monitoring: questions and answers 2004 2nd edGenevaWorld Health Organization [Google Scholar]
[2]. TB CARE I. International Standards for Tuberculosis Care, Edition 3. TB CARE I, The Hague, 2014;43 [Google Scholar]
[3]. Mishra G, Mulani J, Tuberculosis prescription practices in private and public sector in IndiaNational Journal of Integrated Research in Medicine 2013 4(2):71-78. [Google Scholar]
[4]. Mishra G, Ghorpade SV, Mulani J, XDR-TB: an outcome of programmatic management of TB in IndiaIndian J Med Ethics 2014 11:47-52.10.20529/IJME.2014.01324509111 [Google Scholar] [CrossRef] [PubMed]
[5]. World Health Organization. Treatment guidelines for drug resistant tuberculosis: 2016 update. WHO/HTM/TB/2016.04. Geneva, Switzerland: WHO, 2016 [Google Scholar]
[6]. Payen MC, Muylle I, Vandenberg O, Mathys V, Delforge M, Van den Wijngaert S, Meropenem-clavulanate for drug-resistant tuberculosis: a follow-up of relapse-free casesThe International Journal of Tuberculosis and Lung Disease 2018 22(1):34-39.10.5588/ijtld.17.035229297423 [Google Scholar] [CrossRef] [PubMed]
[7]. Central TB Division, Ministry of Health and Family Welfare. Technical and operational guidelines for TB Control in India. New Delhi: Directorate of General Health Services, Ministry of Health and Family Welfare, Government of India; 2016 [Google Scholar]
[8]. Central TB Division, Ministry of Health and Family Welfare. Programmatic Management of Drug Resistant TB (PMDT) in India. New Delhi: Directorate of General Health Services, Ministry of Health and Family Welfare, Government of India; 2017 [Google Scholar]
[9]. Amoxicillin/clavulanate Pediatric Dosing - Epocrates Online [Internet]. Online.epocrates.com. 2018 [cited 17 February 2018]. Available from: https://online.epocrates.com/drugs/41902/amoxicillin-clavulanate/Peds-Dosing [Google Scholar]
[10]. Mishra GP, Caminero JA, Low-dose amoxicillin-clavulanate in drug-resistant tuberculosisThe International Journal of Tuberculosis and Lung Disease 2018 22(4):46510.5588/ijtld.18.005529562998 [Google Scholar] [CrossRef] [PubMed]
[11]. Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, Treatment outcomes and survival based on drug resistance patterns in multidrug-resistant tuberculosisAmerican Journal of Respiratory and Critical Care Medicine 2010 182(1):113-19.10.1164/rccm.200911-1656OC20224066 [Google Scholar] [CrossRef] [PubMed]