Sarcoidosis is a granulomatous disease of unknown origin, that was formerly known as pseudotuberculosis and causes a disease with similar manifestations of Non-Tuberculous Mycobacteria (NTM) [1]. Occurence of sarcoidosis disease is more prevalent in winters and in regions with increased latitude. The immune response to a microbial or chemical agent such as beryllium, zirconium, aluminium, mycobacterium tuberculosis, non-tuberculosis mycobacterium, fungi and spirochetes are among the possible causes of early onset of sarcoidosis symptoms [1-3]. Sarcoidosis affects several organs and the lungs are the main involved organ [2,3]. Sarcoid granulomatous nodes can interconnect with each other and in some cases create larger nodes in the respiratory tract [3,4]. Typically, diagnosis of sarcoidosis relies on a compatible clinical and radiologic presentation in patients presenting with unexplained cough, shortness of breath, or constitutional symptoms. Observing symmetrical prehillar, mediastinal lymphadenopathy, and perilymphatic micronodules in chest X-ray are representative of sarcoidosis. Moreover, increased level of Angiotensin-Converting Enzyme (ACE) in serum of patients is also a known marker for sarcoidosis [2]. Another indispensable diagnostic indicator for sarcoidosis is pathologic evidence of noncaseating granulomas, on involved tissue samples and exclusion of other diseases with similar findings, such as tuberculosis or malignancy. Since the lungs are the main involved organ, pathologic diagnosis is generally examined on the pulmonary biopsy that is obtained by transbronchial lung biopsy [5]. The frequency of Sarcoidosis of the Upper Respiratory Tract (SURT) is estimated to be about 5% of patients with established diagnosis of sarcoidosis. SNS is a form of SURT disease with an incidence of 2% of sarcoidosis cases and was first introduced by Schaumann in 1924 and 1926 [6,7]. Local immune responses that make up the sarcoidosis granuloma are factors that can affect sinonasal destruction and disorder [7]. The non-isolated form of sinonasal granuloma can be seen in 1% of cases. While in most SNS patients, systemic involvement and especially involvements of the lungs are observed [8]. The most common symptoms of SNS cases are nasal obstruction and chronic rhinosinusitis that is mostly unresponsive to at least three week’s conventional treatments [9]. Meanwhile, when diagnosis of SNS cases is based on symptomatology, wrong diagnosis and treatement occur frequently, due to the joint manifestations with diseases such as non-sarcoid rhinosinusitis and allergies [10,11]. Due to the complication of diagnosis and therapeutic issues, SNS patients often are resistant to the initial corticosteroids treatment and mostly require long-term of aggressive systemic treatment [12]. Based on various reports, development of sinonasal involvements vary widely from 1% in isolated form to 15% of sarcoidosis cases [13]. These paradoxical results raise the assumption that many patients with classic symptoms of SNS may be falsely diagnosed. Because the accuracy of symptomatology is uncertain for the diagnosis of SNS, CT scan has been recently used worldwide as the gold standard for the diagnosis of SNS [14]. Attempts to evaluate the prevalence of certain SNS patients will lead to early diagnosis of SNS cases and minimizing unnecessary antibiotic administration in such patients. Moreover, unnecessary surgical treatments in patients with nose and sinus sarcoidosis can therefore be minimized. However, there are very few Iranian case studies that have studied features of sinonasal sarcoidosis [15]. The evaluation of prevalence of SNS cases, in the current survey was investigated using CT scans, for diagnosis. It was assumed that a normal sinus CT excludes the diagnosis of sinonasal involvements. Therefore, as the first report from Iran, the present study was carried out to evaluate the prevalence and to describe the clinical features of patients with sinonasal sarcoidosis in Iran.
Materials and Methods
Study design: The present cross-sectional descriptive study was conducted at sarcoidosis clinic and the rhinology ward between April 2016 and October 2017. Among 608 biopsy proven sarcoidosis patients who attended the clinic at the time of the study, 88 patients with symptomatically suspected sinonasal disease, admitted with complaints of sinonasal symptoms and poorly responsive to antibiotic treatment for at least three weeks. Frequent complaints were: lupus perino [Table/Fig-1], nose deformity, rhinorrhea, anosmia, nasal enlargement, oronasal fistula, cervical lymphadenopathy, salivary gland enlargement, supraglottic nodules and chronic rhinosinusitis.
Clinical presentation of Lupus Perino symptom in a patient.
Inclusion criteria was above 18-year-old biopsy proven sarcoidosis patients who were recently showing head and neck disorders. Smokers, tuberculosis cases and chronic respiratory patients with clinical evidence of other sinonasal granulomatous diseases, such as Wegner disease were excluded from the study.
Eighty eight suspected subjects were evaluated with non-contrast coronal and axial Computed Tomography of Para-nasal Sinuses (CT PNS). CT PNS was normal in sixty six patients that were more likely to show the sinonasal symptoms due to acid reflux and migraine headaches. Involvement of various paranasal sinuses was confirmed in 32 patients. Consequently, 32 sarcoidosis subjects with confirmed-CT paranasal sinuses abnormalities entered the study using a convenience sampling technique. Research project was approved by ethics committee and institutional review board (IR.SBMU.NRITLD.REC.1396.371). Complying with the health indemnification, ethical approval was confirmed to declaration of Helsinki. The principle of secrecy of patient information was observed. Signed consent forms were received from study individuals, after the study procedure was explicated to them.
Data Collection Method
Interviews: Participants were interviewed face-to-face for demographic characteristics including age, gender, nationality. The Persian translated St. George’s Respiratory Questionnaire (SGRQ) was used to collect relevant data regarding sarcoidosis [16].
For the presence of sinonasal symptoms, a self-designed questionnaire based on the sinonasal sarcoidosis standardized diagnosis criteria developed by de Shazo was used to collect data [17].
Diagnosis of pulmonary sarcoidosis: Histopathologic confirmation of non-caseating granuloma in bronchial mucosa biopsy or transbronchial pulmonary biopsy of 58 patients revealed pulmonary involvement of sarcoidosis. Pulmonary biopsy was obtained by Endobronchial Ultrasound (EBUS)-guided Transbronchial Needle Aspiration (TBNA) using a power doppler mode of the EBUS bronchoscope (model BF-UC260FW, EU-ME1 Olympus, Tokyo, Japan), according to the previous guideline [5]. After premedication with Lidocaine, transoral bronchoscopy was done and the biopsy was immediately pulled into sterile polycarbonate tube and sent for further histopathological examinations.
Laboratory tests: Polymerase Chain Reaction (PCR) technique was used for the detection of tuberculosis. To extract DNA from sputum samples, High Pure Nucleic Acid Extraction kit (Roche, Germany) was used. Appropriate specific primers and enzyme were used to perform PCR (Cinnagen, Iran) [18].
Determination of ACE level: Quantification of ACE level was measured in lithium heparin plasma according to previously described methods (Bühlmann Laboratories AG, Switzerland). The manufacturer’s reference interval was 12-68 U/L in above 18 years cases [19].
Clinical tests: All 88 patients were evaluated for chest X-ray and spirometric tests. CT PNS imaging with 16 slice MDCT Bright Speed GE machine was also carried out on all 88 patients. CT PNS confirmed various paranasal sinuses involvement in 32 of 88 patients. Consequently, 32 sarcoidosis subjects with confirmed-CT paranasal sinuses abnormalities were further studied.
Rhinoscopy examination: Afterward, all 32 patients were examined for the Ear, Nose, and Throat (ENT) localizations of sarcoidosis via rhinoscopy. The rhinoscopy examination was performed in the rhinology ward of the same department by a single ENT (ear, nose and throat) surgeon with a 30-degree endoscope (BF-1T260, Olympus Tokyo, Japan) to visualize the main areas in the nasal and sinus cavities, including the nasal floor, nasopharynx, nasal sinuses and the roof of the nose. Prevalent presenting signs of sinonasal involvement were documented and nasal or sinonasal mucosa sample were obtained. Histopathologic examinations of biopsy samples were performed in accordance with the guidelines [7,11,12,20]. Destructive lesions were inspected for excluding other systemic granulomatous diseases such as tuberculosis, Wegener granulomatosis and Crohn’s disease [21,22].
Statistical Analysis
Data were statistically analysed by Statistical Package for Social Sciences (SPSS) (ver. 22.0; SPSS Inc. Chicago, IL, USA) software. Statistical analyses were conducted following international statistical standards. Descriptive statistical indicators, including frequency and percentage for qualitative variables were used. Continuous variables were expressed as mean±standard deviation. Differences between two groups were analysed using the χ2 test for categorical data, whereas the Mann-Whitney test and independent t-test were used for continuous variables. A p-value of <0.05 was considered to be statistically significant.
Treatment: Sixty six patients with normal CT PNS results received oral non-steroidal anti-inflammatory treatment. In the case of 32 SNS patients, based on the severity of disease, systemic corticosteroids treatment was prescribed solely (twenty two cases) or combined with local corticosteroids (nine cases) according to former studies [23]. The dose of prednisolone was 0.5 to 1 mg/kg with a gradual reduction. Two patients refused to take any form of corticosteroids. Thus, Hydroxychloroquinine was the alternate treatment suggested in them. In four patients, nasal septoplasty and sinus surgery were performed in addition to systemic corticosteroids therapy. Intensive treatment with Methotrexate (MTX) was prescribed in two patients. The duration of treatment varied from six months to one year. Recurrence of sinonasal symptoms were evaluated in all patients, six months after the recovery.
Results
Demographic and disease characteristics: A total of 88 patients were primarily included in this study. All patients were Iranian and white. The mean age of the patients was 47.44 years with a female to male ratio of 1.6:1. Among the studied cases, 55 (62.5%) were women and 33 (37.5%) were men, with ages ranging from 20 to 75 years (mean age, 47.44±9.07). [Table/Fig-2] presents the data of the demographic characteristics of the 88 subjects.
Demographic characteristics of 88 SNS suspected subjects.
| Sinonasal suspected sarcoidosispatients (n) | 88 |
| Age, years mean (range) | 47.44 yr±9.07 (20-75) |
| Male/female | 1/1.6 |
| Pulmonary involvement | 58 |
Complaints: The most frequently mentioned complaints by the patients were congested mucosa, erythema nodosum, deformity of nose and smell disorder. A total of 58 pulmonary confirmed sarcoidosis patients with chest X-rays radiography were staged based on the Scadding criteria as stage I (n=4) and stage II (n=54). Furthermore, the rest of patients demonstrated normal chest radiography. The tuberculosis PCR tests were negative in sputum sample of all 88 patients and all patients had normal spirometry findings.
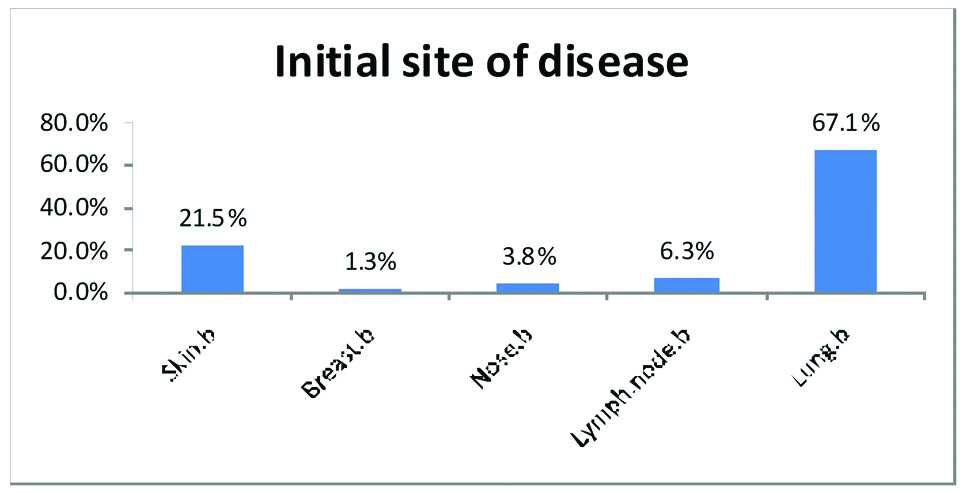
The primary site of occurrence of sarcoidosis which was also proved by biopsy was; lymph node in 6 of 88 patients (6.3%), nose in 3 of 88 patients (3.8%), breast in 1 of 88 patients (1.3%), lung in 58 of 88 patients (67.1%), skin in 19 of 88 patients (21.5%) and two cases with simultaneous skin and lung involvements [Table/Fig-3].
The initial site of sarcoidosis disease.
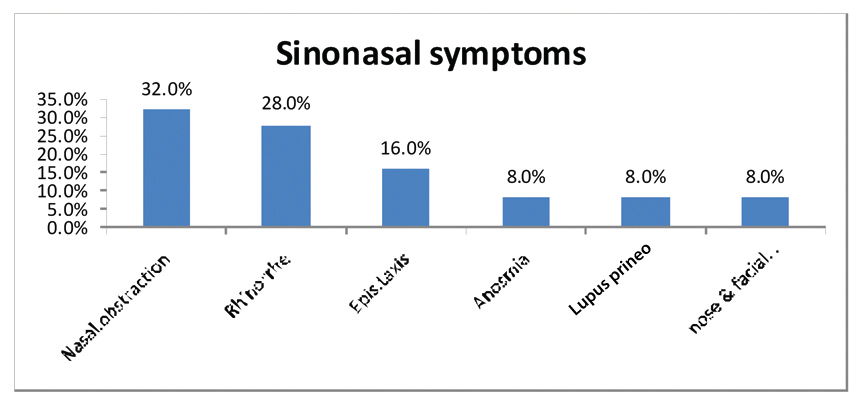
Nasal symptoms and endoscopic findings: CT PNS in 88 patients confirmed signs of chronic sinus inflammation in 32 patients. All of 32 patients were symptomatic and the most common symptoms were; nasal obstruction in 11 patients (32.0%), rhinorrhea in 10 patients (28.0%), epistaxis in 5 patients (16.0%), anosmia in 3 patients (8%), lupus prineo in 3 patients (8%) and nose and facial deformity in 3 patients (8%). However, none of the patients showed oronasal fistula symptoms [Table/Fig-4]. The interval time between demonstrating sinonasal symptoms and the diagnosis of SNS varied widely ranging from one month to two years.
The most common presented symptoms in sarcoidosis patients.
CT PNS, confirmed symptoms of 32 patients, consisted of mucosal thickening, Para-nasal sinusitis, septal perforation, deformity of nose, septal submucosal nodules. However, opacification of sinuses was not observed in any of the cases. The involvement of the maxillary sinuses in current patients was more frequent than ethmoidal sinus or frontal sinus. All of the 32 confirmed-CT SNS subjects, who participated in the study, completed the study and none of them left the study.
Clinical findings: ACE above 80 was obtained in laboratory findings of 32 patients with a mean level of 72.96±44.80. The mean level of ESR was 28.80±28.70. Hyperthyroidism comorbidity factor was not observed in any cases. The median ACE in 32 patients with nasal mucosal abnormalities was noted to be significantly higher than the patients with normal nasal endoscopic findings (p=0.01). However, the median ACE of the patients with nasal symptoms, although higher than the patients without nasal symptoms (1 versus 0), was not statistically significant.
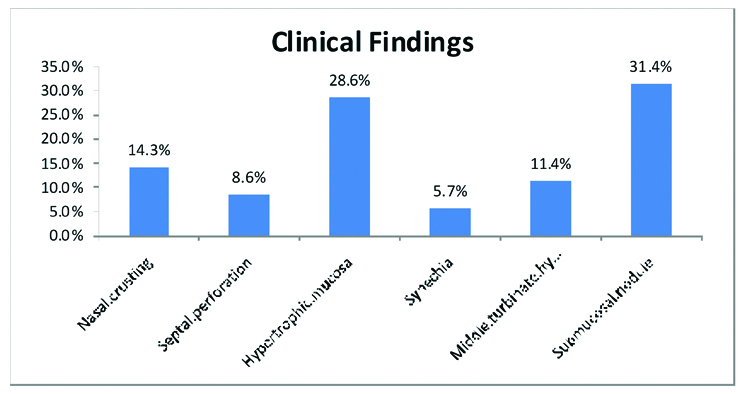
Rhinology examination findings: Sinonasal abnormalities were observed in rhinoscopy examinations of all 32 patients. Histopathological examinations of nasal or sinusal mucosa biopsy sample showed sarcoidal non-caseating granulomatous lesions in all 32 cases. The most observed clinical findings on rhinoscopic investigations were: nasal crusting in 4 patients (14.3%), septal perforation in 3 patients (8.6%), mucosal hypertrophy in 9 patients (28.6%), synechia in 2 patients (5.7%), middle turbinate hypertrophy in 4 patients (11.4%) and submucosal nodule in 10 patients (31.4%) [Table/Fig-5]. However, no appreciable association was seen between the presence of any of nasal symptoms and nasal mucosal abnormalities on endoscopy (χ2, p>0.05). In none of the patients, the sinonasal involvement was the primary cause of sarcoidosis and it was developed in the years after the diagnosis of sarcoidosis.
The most common sinonasal abnormalities and signs in studied sarcoidosis patients.
Multiorgan SNS: Two isolated SNS patients (6.25% of 32 SNS patients) were obtained in the current study. Concomitant of sinonasal and pulmonary organs was confirmed in 18 of 32 SNS patients (56.25%). Concomitant of sinonasal and lymph node, skin and breast involvements were confirmed in 4 of 32 SNS patients (12.5%), 7 of 32 SNS patients (21.87%), 1 of 32 SNS patients (3.12%) respectively.
Treatment and follow-up: Combined local corticosteroids with systemic treatment consisted of nasal and inhalation form of steroids prescribed in nine cases. Nine patients that received systemic corticosteroids treatment combined with local corticosteroids reported greater improvements in symptoms. Nasal septoplasty and sinus surgery outcomes were satisfactory in all four patients. Two patients who only received corticosteroids exhibited treatment failure. Intensive treatment with MTX resulted in treatment improvements. Follow-up of three patients were not possible, due to not referring to the clinic. In current experience, all the SNS patients showed satisfactory symptoms improvement, after 9.2 months. Response rate to treatment was variable from partial improvement to complete remission in six month follow-up.
Discussion
Despite the fact that the parotid paralysis of the facial nerve is one of the primary symptoms of head and neck sarcoidosis, sinonasal involvement, is a rare form of sarcoidosis disease [20]. As the first report from Iran, the present study was carried out to evaluate the prevalence and to describe the clinical features of patients with sinonasal sarcoidosis in Iran.
In the current study, the frequency of biopsy proven sinonasal sarcoidosis patients were about 5.26% (32/608) of total studied cases, although, the frequency of upper airway symptomatically suspected cases was higher and 14.47% (88/608) of total sarcoidosis patients. This finding suggests that a symptomatic approach for diagnosis of sinonasal manifestation in sarcoidosis, compared to CT and endoscopic investigations is not efficient (5.26% vs 14.47%). On this basis, the overall incidence of sinonasal involvements was 5.26% (32/608) among biopsy proven sarcoidosis patients. This frequency was higher than some previous studies that reported a slightly less than one percent of affected patients by sinonasal involvement [7,14]. These varied data suggest that the prevalence of nasal abnormalities may differ significantly from one population to another due to genetic variations and ethnic diversity.
In the current study the majority of patients developed sinonasal involvement after the initial diagnosis of sarcoidosis. In contrast to this, a recent case-control study of 20 patients showed that sinonasal involvement resulted in the diagnosis of sarcoidosis in the majority of the patients [7].
The current study demonstrated that generalized disease is common among patients with sinonasal sarcoidosis (93.75% of 32 SNS patients). This finding is in line with recent data that showed multi-organ involvement in 66.66% of SNS studied cases [7].
However, another research reported a rarely frequency of generalized organ involvement in the sarcoidosis patients with laryngeal involvement [6]. Since, in the current study we only investigated the head involvement in sarcoidosis patients, further studies on head and neck involvements with a focus on the laryngeal involvement are suggested in sarcoidosis patients of Iran.
According to former studies, CT PNS can offer some evidences that help to distinguish criteria of SNS [7]. CT scans of the paranasal sinuses in primary 88 suspected patients showed a predominant nasal obstruction and rhinorrhea in studied patients, whereas some signs of chronic sinus inflammation that were described in some other studies, like oronasal fistula were not seen in any of studied cases [7]. In contrast to current observation, another previous study on nasal involvement in SNS patients, mentioned the mucosal thickening and sinus opacification as the frequently CT findings [24]. Differences in the results may be due to studied populations’ diversity.
Current endoscopic investigations indicated that the most frequent manifestation of SNS cases was inflammatory rhinosinusitis often with nodular lesions on the septum and/or turbinates. Also, destructive crusting occasionally is observed in the studied group. Biopsy of mucosa provided a high diagnosis capability, with positive results in all 32 cases. In the current study, the frequency of hypertrophic nasal mucosal abnormalities was obtained 28.6% (9 of 32 cases) and 10.22% of 88 symptomatic suspected subjects. However, no appreciable association was seen between the presence of any of nasal symptoms and nasal mucosal abnormalities on endoscopy (χ2, p>0.05). Current findings were consistent with other former studies that failed to show a link between disease activity and the presence of nasal symptoms and nasal mucosal abnormalities [25]. However, since the nasal obstruction was the commonest sinonasal problems in Iranian patients, further researches are recomended in order to measure the sensitivity and specificity of nasal obstruction as a diagnostic parameter.
In current study, the median ACE in 32 patients with nasal mucosal abnormalities was noted to be significantly higher than the patients with normal nasal endoscopic findings (p=0.01). However, the median ACE of the patients with nasal symptoms, although higher than the patients without nasal symptoms (1 versus 0), was not statistically significant.
In some previous studies, endoscopic sinus surgery was recommended to be prescribed in addition to systemic treatment in SNS cases [16,20]. However, in current study, surgical interventions were only suggested in the patients with no improvements in the symptoms during the systemic therapy. Although recent studies describe local topical treatment of sinonasal sarcoidosis as inadequate, the current study obtained the satisfactory results of topical treatment for SNS patients [7,22]. Nine patients who received corticosteroids treatment combined with local corticosteroids showed more symptom improvements. Results of the current study showed that the intensive treatment with MTX appears to be impressive in patients with treatment failure.
Limitation
Since, this study was carried out using samples collected from the referral hospital for lung diseases in Tehran; therefore it may not reflect the overall country scenario. Finally, despite the current study was conducted on a large cohort, but it was influenced by some limitations. It is suggested that, biger sample size of patients in future studies may help in more accurate generalization of the results.
Conclusion
To conclude, sinonasal involvement is a rare disease manifestation of sarcoidosis. The current study suggests that a symptomatic approach for diagnosis of sinonasal manifestation in sarcoidosis is not efficient. Hence, the key to the certain diagnosis of sinonasal manifestation in sarcoidosis is to perform CT in suspicious cases. In the case of observing abnormal pattern in CT, plus increased ACE level in a sarcoidosis patient, further histopathological examination on sinonasal biopsy is suggested. However, further studies with focus on head, neck and laryngeal involvement would be necessary to evaluate the exact prevalence of head and neck involvements in sarcoidosis patients in Iran.