Introduction
Immune system refers to the assortment of defence cells against foreign antigens. The line of defence in the immune system encompasses Innate and Adaptive immune responses [1]. D2-Immune system is under the control and regulation of inflammatory cells which safeguard and prevent body from cellular injury and infections. Inflammatory cells can also act as a “double-edged sword” in both protective and destructive manner emphasizing the role of immune cells in autoimmune mechanism wherein self antigens are recognised as antigenic. Instant recognition of encroaching foreign antigen is perceived by innate immune mechanism which is deprived of immunological memory. Adaptive immune response is antigen dependent and specific with immunologic memory which empowers immune mechanism upon succeeding antigenic response [2]. Central intra-thymic deletion of self-reactive T lymphocytic cells which own receptors for self-antigen is self-tolerance [3]. Inhibition of self-antigen reactive immune cells in the periphery is performed by regulatory T-cells (T regs) [4,5]. Some T cells termed as ignorant T-cells are neither deleted in the periphery nor in the thymus due to the diminished amount of their cryptic antigen [6]. D3-Antigen Presenting Cells (APC) along with surface Major histocompatibility complex present antigen to T-lymphocytes. CD4+T cells are restricted by MHC class II antigens and CD8+ cells are restricted by MHC class I antigens. Major histocompatibility (MHC) complex comprising HLA (Human Leukocyte Antigen) class I and HLA class II could perceive foreign antigens and present to T-lymphocytic receptors [7]. Self Non-Self (SNS) model, Infection Non-Self (INS) model, Danger model and Two Signal model are considered as some of the ideal concepts of immune reactions [8]. Autoimmune disease is the sequelae of impairment of self-tolerance to self-antigen.
Autoimmune diseases are exceeding 100 million people globally [9]. Cessation of central and peripheral tolerance is found to be associated with prolonged environmental insults generating immune response against self-antigens. Molecular mimicry, bystander activation, cryptic antigenic and super antigenic exposure can promote autoimmune reactions [10-12].
Concepts in Autoimmunity
Self Non-Self model theory and Two Signal model theory: The SNS model theory of immunity states that any non-self or foreign antigen can trigger immune mechanism in the host, whereas self endogenous elements will not provoke an immune reaction in the host. Confrontation of body with any foreign material can bring about immune response. A contrast statement was proposed by Jerne which recommends that a constant negligible reaction should be there by immune cells on self molecules for normal immune surveillance [13]. During lymphocytic selection in the thymus or bone marrow, these immune cells could survive in the primary lymphoid organs only if it reacts on self molecule. Deprivation of weak reaction of circulating lymphocyte on self antigen can result in its cessation. T regulatory lymphocytes which are self immune cells control the normal self lymphocytes during immune functions [14-16]. Immune tolerance of host immune cells to gut bacteria, certain helminths, alloantigenic graft, chimerism and foetomaternal tolerance are countering SNS model theory of immunology [17-20].
In the initial phase of SNS model, it was postulated that interaction of B-cell antigenic receptors and T-cell antigenic receptors with an antigen can generate immune reactions. Subsequently two signal model theory of immunology was implied, declaring that B-cell after processing antigen, re-express it on MHC class II molecule for T-helper cells to identify [21,22]. A consequent theorization was proposed recommending that T-lymphocytes need stimulatory signals from Antigen Presenting Cells (APC) for immune reaction. TCR signalling is monitored by co-stimulatory and co-inhibitory receptors on its surface. After the recognition of antigenic peptides presented by MHC molecules to T-Cell Receptors (TCR), T-cell function and fate is determined by the co-stimulatory and co-inhibitory receptors on T-cells.
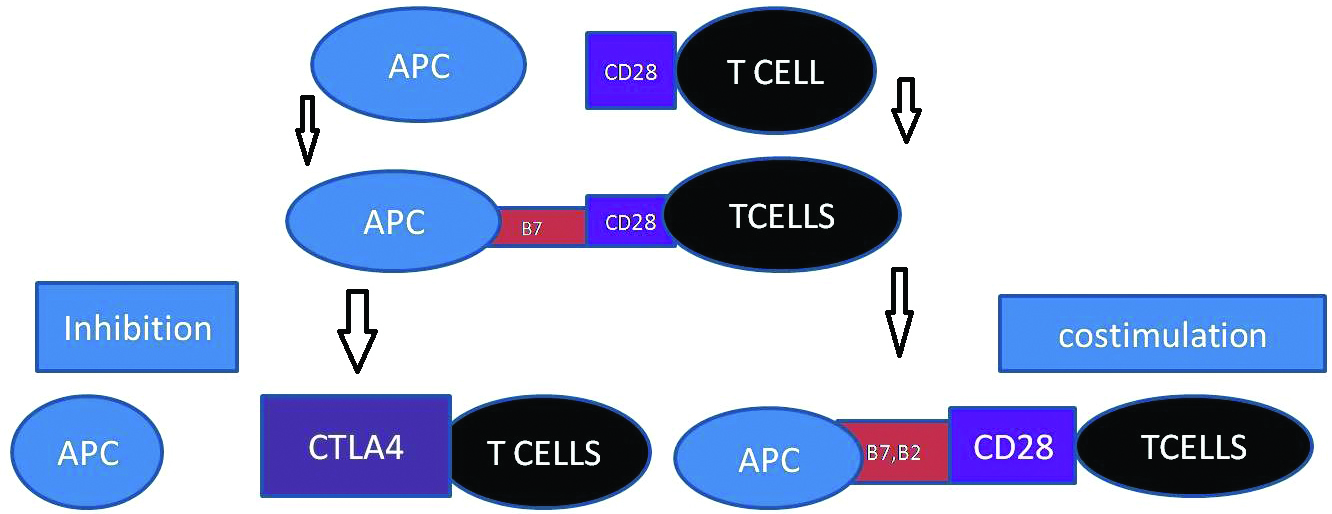
According to the two signal hypothesis of immunity, both antigen and secondary stimuli are required for T cell activation. The most complex two signal model, immune regulatory system operates with the conjugation of the co-stimulatory receptor CD28 (ligand, B7-1), and co inhibitory receptor (Cytotoxic T Lymphocyte Antigen-4 (CTLA4), which also binds to B7-1) and a second ligand (B7-2, which binds to both CD28 and CTLA4) [23-25]. Expression of B7-1 and B7-2 is modulated by the activation state of the APC. T cell growth and survival is promoted by CD28 co stimulatory receptor, expressing on the cell surface of naive CD4+ and CD8+T cells upon ligation by B7-1 and B7-2 on antigen-presenting cells (APCs) [26-28]. Instigation of CTLA4, co inhibitory receptor is promoted by the activation of T cell and increased expression of CTLA4 declines CD28 expression by endocytosis [29-31]. Cellular insults activate APCs and induce transcription, translation and transportation of both B7-1 and B7-2 to the cell surface [32,33]. The formation of the immune synapse allows TCR signalling and co-signalling [34,35] [Table/Fig-1] D1. The central, peripheral and distal Supra-Molecular Activation Complexes (cSMAC, pSMAC and dSMAC) form the immune synapse with TCR which provides evidence for the two-signal model of T cell activation [36,37]. Co signalling ligands and counter-receptors are highly expressed on the professional Antigen Presenting Cells (APCs).
Two signal model hypothesis.
APC: Antigen presenting cells, CD28 (T cells co-stimulating receptor, B7 and B2 (co-stimuating receptor of antigen presenting cell, CTLA4 (co-inhibiting receptor of T cell
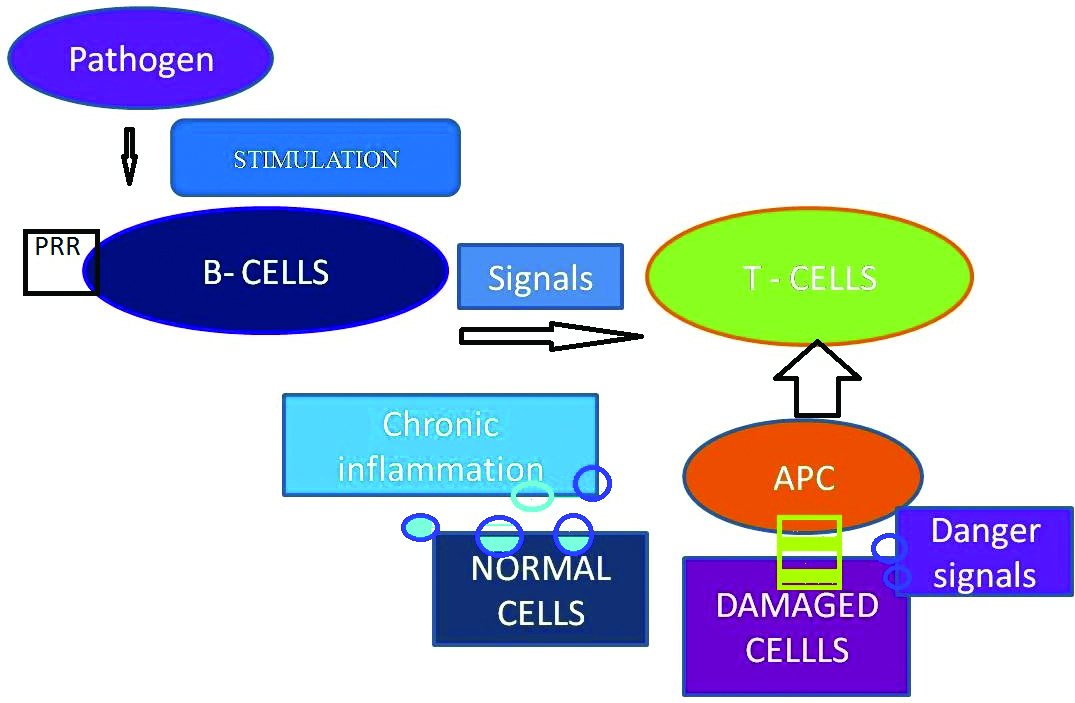
Danger model theory: Danger model hypothesis was postulated by Polly Matzinger, according to which the immune system is more concerned with safe guarding the individual from insults rather than discriminating self and non-self. Circumstances like Mechanical stress, DNA damage, heat, cold and hypoxia can result in cellular damage. Injured, distressed, damaged or necrotic cells could liberate danger or alarm signals which stimulate APC whereas healthy cells send calm signals and apoptotic cells convey eat me signals to APC [38-40]. Repeated cellular insults generate danger alarm signals augmenting antigen presentation which in due course recognises, self protein antigenic, precipitating chronic autoimmunity D2 [Table/Fig-2] [41,42]. A S8-Some of the Endogenous signals are Heat Shock Proteins (HSP), hyaluronan, High Mobility Group Box-1 Mediator (HMGB1), Fibronectin fragments, modified low density lipoprotein and extracellular ATP [43-48].
Danger model theory.
PRR: Pattern recognising receptors; APC: Antigen presenting cells
HSP which are released on account of cellular stress could switch on innate immune cell via Toll-Like Receptors (TLRs). HSP induces the production of nitric oxide (NO), down regulation of tumour necrosis factor α, activates IL12 and regulatory T-cells and improves antigen presentation if associated with antigen [49-51]. The sulphated glycosaminoglycans, hyaluronan which is a major component of extracellular matrix is cleaved to hyaluronic acid during tissue injury [52,53]. This signalling response could institute innate immune response and propagation of dendritic cell maturation contributing adaptive immune response. HMGB1 is released from necrosed cells with the aid of macrophages and dendritic cells. HMGB1 with its endogenous stimulatory mechanism can contribute autoimmune reaction with existing chronic inflammation [54,55]. Fibronectin fragments, defensin and modified low density lipoprotein through divergent immune response can contribute to immune reaction.
Immunopathology of Autoimmune Reactions
Molecular Mimicry: Elicitation of immune response against self antigens, when there exist an antigenic resemblance between foreign peptide or pathogen and self peptide is called molecular mimicry [56]. The central and peripheral tolerance which is specifically the positive and negative selection of primitive T cells occurs in the thymus [57]. The selection depends on the competence of T cells to interact with the peptides presented by MHC. T-cells, with negative selection of MHC presented peptides and strong interactions with self antigens are nullified [58,59]. Instead in molecular mimicry a constant immune response against self-antigens is ensued, when the pathogen has a structural and sequential resemblance with self antigens [Table/Fig-3]. Molecular mimicry can also be emerged by virtue of neoepitope formation acquiring via tissue injury. Tissue injury can contribute to the development of self reactive T cells by exposing the self cryptic epitopes [60,61].
Molecular mimicry.
APC: Antigen presenting cells; T cells: T lypmbocyles
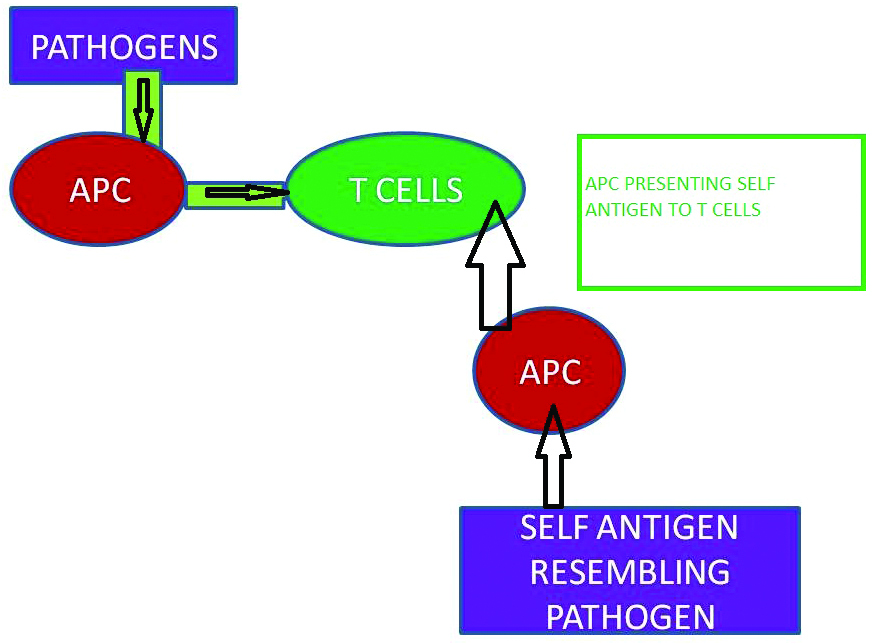
AS10-TCR is poly specific in nature. The structural resemblance of pathogens to self antigens creates dilemma to TCR inducing molecular mimicry [62]. A foreign peptide (such as virus) resembling MHC derived peptide can activate T cells, and if the self antigen is also structurally similar, exposure to the foreign peptide results in these activated T cell becoming autoreactive [63,64]. Molecular mimicry is confederated to several autoimmune diseases like Multiple sclerosis, Gullian-Barre syndrome, Acute Rheumatic fever, type 1 Diabetes Mellitus, Lyme arthritis and Ankylosing Spondylitis. A structural homology between Epstein-Barr virus and Myelin basic protein was evident in multiple sclerosis [65]. In Gullian–Barre syndrome post infectious molecular mimicry between nerve antigen and microbes induce autoimmune response [66]. Epitope mimicry between Streptococcal M proteins and human myosin, tropomyosin, keratin, actin, laminin, vimentin and N–acetylglucosamine contribute to acute rheumatic fever [67]. In type 1 diabetes mellitus, mimicry related to viral infection is hypothesized due to the structural similarity between pancreatic beta cell enzyme and viruses [68,69].
Bystander Activation: In bystander activation, viral and bacterial products induce INF α/β (Interferon alfa/beta) secretion which propagates the proliferation and expansion of heterologous polyclonal T cells [70]. Activation of INF α/β D11 (Interferon alfa /beta) leads to the stimulation of APC [71]. APC produce the cytokine, IL -15 which then activates memory phenotype (MP CD+8) T cells. CD8+T cells recognize the infected cells and release cytokines like TNF (Tumour Necrosis Factor), NO (Nitric Oxide) and Lymphocyte Toxins (LT) resulting in the causation of bystander killing of uninfected neighbouring cells D3 [72]. Cytokines released from CD4+T cells can directly kill uninfected cell but the bystander activation of CD4+T cells are less competent than CD8+T Cells. CD 122 receptor for IL2/IL15 is more expressed by MP CD8+T cells than MP CD4+T which results in limited bystander activation of MP CD4+T [73].
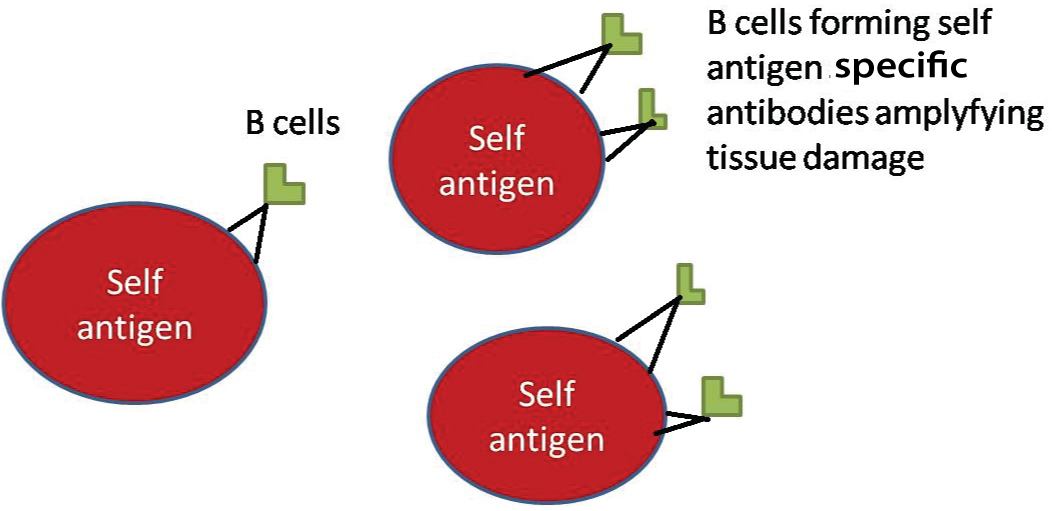
Epitope Spreading: AS13-Epitope spreading can be defined as a specific autoreactive lymphocyte (T or B cell) response to endogenous epitopes, secondary to the release of such self protein during a chronic autoimmune or inflammatory response (Vanderlugt and Miller, 1996) [Table/Fig-4] [74]. Cell mediated and humoral immunities are influenced in epitope spreading by the unmasking of multiple epitopes on a single antigen or from one antigenic molecule to other. The biological events elucidated in epitope spreading include endocytic processing, antigen presentation, and somatic hypermutation. B cell epitope spreading appeared to be inveigled in autoimmune diseases like Systemic lupus erythematous, multiple sclerosis, pemphigus and bullous pemphigoid [75]. T cell epitope spreading is accomplished in Multiple Sclerosis and Autoimmune Encephalomyelitis, by a concurrent regression of primary autoreactivity associated with disease onset [76].
Epitope spreading showing clonal expansion of autoantibodies causing damage on self antigens.
Conclusion
Insight on immune pathways, impaired immunity and evolution of autoimmune diseases can supplement on the therapeutic modalities of immunological diseases. Distraction in the suppression of the immune response to the self antigens contributes to autoimmune diseases. Various mechanisms, including the release of sequestered antigens, molecular mimicry, epitope spreading, cytokine deregulation and inappropriate expression of class II MHC molecules propagate autoimmunity.
[1]. Turvey SE, Broide DH, Innate immunityJ Allergy Clin Immunol 2010 125(2):24-32.10.1016/j.jaci.2009.07.01619932920 [Google Scholar] [CrossRef] [PubMed]
[2]. Sun JC, Ugolini S, Vivier E, Immunologicalmemory within the innate immune systemEMBO J 2014 33(12):1295-303.10.1002/embj.201387651 [Google Scholar] [CrossRef]
[3]. Griesemer AD, Sorenson EC, Hardy MA, Role of thymus in toleranceTransplantation 2010 90(5):465-74.10.1097/TP.0b013e3181e7e54f20555306 [Google Scholar] [CrossRef] [PubMed]
[4]. Sakaguchi S, Wing K, Miyara M, Regulatory T cells-a brief history and perspectiveEur J Immunol 2007 37:116-23.10.1002/eji.20073759317972355 [Google Scholar] [CrossRef] [PubMed]
[5]. Sojka DK, Huang YH, Fowell DJ, Mechanisms of regulatory T-cell suppression–a diverse arsenal for a moving targetImmunology 2008 124:13-22.10.1111/j.1365-2567.2008.02813.x18346152 [Google Scholar] [CrossRef] [PubMed]
[6]. Kurts C, Sutherland RM, Davey G, Li M, Lew AM, Blanas E, CD8 T cell ignorance or tolerance to islet antigens depends on antigen doseProc Natl Acad Sci U S A 1999 96(22):12703-07.10.1073/pnas.96.22.1270310535986 [Google Scholar] [CrossRef] [PubMed]
[7]. Miles JJ, McCluskey J, Rossjohn J, Gras S, Understanding the complexity and malleability of T-cell recognitionImmunology and Cell Biology 2015 93:433-41.10.1038/icb.2014.11225582337 [Google Scholar] [CrossRef] [PubMed]
[8]. Matzinger P, The danger model: a renewed sense of selfScience 2002 296:301-05.10.1126/science.107105911951032 [Google Scholar] [CrossRef] [PubMed]
[9]. Cooper GS, Bynum ML, Somers EC, Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseasesJ Autoimmun 2009 33(3-4):197-207.10.1016/j.jaut.2009.09.00819819109 [Google Scholar] [CrossRef] [PubMed]
[10]. Fujinami RS, Oldstone MBA, Amino acid homology between the encephalitogenic site of myelin basic protein and virus: Mechanism for autoimmunityScience 1985 230:1043-45.10.1126/science.24148482414848 [Google Scholar] [CrossRef] [PubMed]
[11]. Oldstone MBA, Molecular mimicry and autoimmune diseaseCell 1987 50:819-20.10.1016/0092-8674(87)90507-1 [Google Scholar] [CrossRef]
[12]. McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD, Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitisJ Exp Med 1995 182:75-85.10.1084/jem.182.1.757540658 [Google Scholar] [CrossRef] [PubMed]
[13]. Pradeu T, Carocella ED, The self model and the conception of biological identity in immunologyBiology and Philosophy 2006 21:235-52.10.1007/s10539-005-8621-6 [Google Scholar] [CrossRef]
[14]. Loh DY, Sha WC, Nelson CA, Newberry RD, Kranz Russell JH, Positive and negative selection of T lymphocytesCold Spring Harb Symp Quant Biol 1989 54:147-51.10.1101/SQB.1989.054.01.0182639750 [Google Scholar] [CrossRef] [PubMed]
[15]. Goodnow CC, Balancing immunity and tolerance: deleting and tuning lymphocyte reperpetoiresProc Natl Acad USA 1996 93(6):2264-71.10.1073/pnas.93.6.22648637861 [Google Scholar] [CrossRef] [PubMed]
[16]. Hoyne GF, Lamb JR, Regulation of T cell function in mucosal toleranceImmunology and Cell Biology 1997 75:197-201.10.1038/icb.1997.299107576 [Google Scholar] [CrossRef] [PubMed]
[17]. Berg RD, The indigenous gastrointestinal microfloraTrends in Microbiology 1996 4:430-35.10.1016/0966-842X(96)10057-3 [Google Scholar] [CrossRef]
[18]. Buscaglia CA, Di Noia JM, Trypanosoma cruzi clonal diversity and the epidemiology of Chagas’ diseaseMicrobes Infect 2003 5(5):419-27.10.1016/S1286-4579(03)00050-9 [Google Scholar] [CrossRef]
[19]. Ferguson TA, Griffith TS, A vision of cell death: insights into immune privilegeImmunological review 1997 156:167-84.10.1111/j.1600-065X.1997.tb00967.x9176707 [Google Scholar] [CrossRef] [PubMed]
[20]. Aluvihare VR, Regulatory T cells mediate maternal tolerance to the fetusNat Immunol 2004 5(3):266-71.10.1038/ni103714758358 [Google Scholar] [CrossRef] [PubMed]
[21]. Langman RE, Cohn M, Self-non-self discrimination revisitedSemin Immunol 2000 12:159-62.10.1006/smim.2000.022710910734 [Google Scholar] [CrossRef] [PubMed]
[22]. Cohn M, Langman RE, The protection: the unit of humoral immunity selected by evolutionImmunol Rev 1990 115:11-147.10.1111/j.1600-065X.1990.tb00783.x2202659 [Google Scholar] [CrossRef] [PubMed]
[23]. Linsley PS, CTLA-4 is a second receptor for the B cell activation antigen B7J Exp Med 1991 174:561-69.10.1084/jem.174.3.5611714933 [Google Scholar] [CrossRef] [PubMed]
[24]. Azuma M, B70 antigen is a second ligand for CTLA-4 and CD28Nature 1993 366:76-79.10.1038/366076a07694153 [Google Scholar] [CrossRef] [PubMed]
[25]. Hathcock KS, Identification of an alternative CTLA-4 ligand costimulatory for T-cell activationScience 1993 262:905-07.10.1126/science.76943617694361 [Google Scholar] [CrossRef] [PubMed]
[26]. Knieke K, Lingel H, Chamaon K, Brunner-Weinzierl MC, Migration of Th1 lymphocytes is regulated by CD152 (CTLA-4)-mediated signaling via PI3 kinase-dependent Akt activationPlos One 2012 7:1-9.10.1371/journal.pone.003139122412835 [Google Scholar] [CrossRef] [PubMed]
[27]. Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ, Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and functionJ Exp Med 1994 180:631-40.10.1084/jem.180.2.6317519245 [Google Scholar] [CrossRef] [PubMed]
[28]. Boomer JS, Green JM, An enigmatic tail of CD28 signalingCold Spring Harb Perspect Biol 2010 2:01-12.10.1101/cshperspect.a00243620534709 [Google Scholar] [CrossRef] [PubMed]
[29]. Janardhan SV, Praveen K, Marks R, Gajewski TF, Evidence implicating the Ras pathway in multiple CD28 costimulatory functions in CD4+T cellsPlos One 2011 6:01-10.10.1371/journal.pone.002493121949793 [Google Scholar] [CrossRef] [PubMed]
[30]. Saito T, Yokosuka T, Hashimoto-Tane A, Dynamic regulation of T cell activation and co-stimulation through TCR-microclustersFEBS Lett 2010 584:4865-71.10.1016/j.febslet.2010.11.03621110974 [Google Scholar] [CrossRef] [PubMed]
[31]. Koshland DE, Recognising self from non selfScience 1990 248:127310.1126/science.23564622356462 [Google Scholar] [CrossRef] [PubMed]
[32]. Matzinger P, Tolerance, danger, and the extended familyAnnu Rev Immunol 1994 12:99110.1146/annurev.iy.12.040194.0050158011301 [Google Scholar] [CrossRef] [PubMed]
[33]. Rock KL, Kono H, The inflammatory response to cell deathAnnu Rev Pathol 2008 3:99-126.10.1146/annurev.pathmechdis.3.121806.15145618039143 [Google Scholar] [CrossRef] [PubMed]
[34]. Davis DM, Dustin ML, What is the importance of the immunological synapse?Trends Immunol 2004 25:323-27.10.1016/j.it.2004.03.00715145322 [Google Scholar] [CrossRef] [PubMed]
[35]. Thauland TJ, Parker DC, Diversity in immunological synapse structureImmunology 2010 131:466-72.10.1111/j.1365-2567.2010.03366.x21039474 [Google Scholar] [CrossRef] [PubMed]
[36]. Dustin ML, Chakraborty AK, Shaw AS, Understanding the structure and function of the immunological synapseCold Spring Harb Perspect 2010 2(10):00231110.1101/cshperspect.a00231120843980 [Google Scholar] [CrossRef] [PubMed]
[37]. Griffiths GM, Tsun A, Stinchcombe JC, The immunological synapse: a focal point for endocytosis and exocytosisJ Cell Biol 2010 189:399-406.10.1083/jcb.20100202720439993 [Google Scholar] [CrossRef] [PubMed]
[38]. Matzinger P, Friendly and dangerous signals: is the tissue in control?Nat Immunol 2007 8:11-13.10.1038/ni0107-1117179963 [Google Scholar] [CrossRef] [PubMed]
[39]. Matzinger P, The evolution of the danger theoryExpert Rev Clin Immunol 2012 8:311-17.10.1586/eci.12.2122607177 [Google Scholar] [CrossRef] [PubMed]
[40]. Matzinger P, Kamala T, Tissue-based class control: the other side of toleranceNat Rev Immunol 2011 11:221-30./10.1038/nri294021350581 [Google Scholar] [CrossRef] [PubMed]
[41]. Akira S, Uematsu S, Takeuchi O, Pathogen recognition and innate immunityCell 2006 124:783-801.10.1016/j.cell.2006.02.01516497588 [Google Scholar] [CrossRef] [PubMed]
[42]. Akira S, Takeda K, Toll-like receptor signallingNat Rev Immunol 2004 4:499-511.10.1038/nri139115229469 [Google Scholar] [CrossRef] [PubMed]
[43]. Kaufmann SH, Heat shock proteins and the immune responseImmunol Today 1990 11:12910.1016/0167-5699(90)90050-J [Google Scholar] [CrossRef]
[44]. Van Eden W, Van der Zee R, Paul AG, Prakken BJ, Wendling U, Anderton SM, Do heat shock proteins control the balance of T-cell regulation in inflammatory diseases?Immunol Today 1998 19:30310.1016/S0167-5699(98)01283-3 [Google Scholar] [CrossRef]
[45]. Scheibner KA, Hyaluronan fragments act as an endogenous danger signal by engaging TLR2J Immunol 2006 177:1272-81.10.4049/jimmunol.177.2.127216818787 [Google Scholar] [CrossRef] [PubMed]
[46]. Kovacs-Simon A, Titball RW, Michell SL, Lipoproteins of bacterial pathogensInfect Immun 2011 79(2):548-61.10.1128/IAI.00682-1020974828 [Google Scholar] [CrossRef] [PubMed]
[47]. Vohra RS, Murphy JE, Walker JH, Ponnambalam S, Homer-Vanniasinkam S, Atherosclerosis and the Lectin-like Oxidized low-density lipoprotein scavenger receptorTrends Cardiovasc Med 2006 16:60-64.10.1016/j.tcm.2005.12.00116473764 [Google Scholar] [CrossRef] [PubMed]
[48]. Miller YI, Viriyakosol S, Binder CJ, Feramisco JR, Kirkland TN, Witztum JL, Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cellsJ Biol Chem 2003 278:1561-68.10.1074/jbc.M20963420012424240 [Google Scholar] [CrossRef] [PubMed]
[49]. Ohshima H, Bartsch H, Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesisMutat Res 1994 305:253-64.10.1016/0027-5107(94)90245-3 [Google Scholar] [CrossRef]
[50]. Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, Tak PP, Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signalingJ Immunol 2009 182:4965-73.10.4049/jimmunol.080156319342676 [Google Scholar] [CrossRef] [PubMed]
[51]. Singh IS, He JR, Calderwood S, Hasday JD, A high affinity HSF-1 binding site in the 5’-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressorJ Biol Chem 2002 277(7):4981-88.10.1074/jbc.M10815420011734555 [Google Scholar] [CrossRef] [PubMed]
[52]. Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4J Exp Med 2002 195:99-111.10.1084/jem.2000185811781369 [Google Scholar] [CrossRef] [PubMed]
[53]. Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR, Hyaluronan fragments act as an endogenous danger signal by engaging TLR2J Immunol 2006 177:127210.4049/jimmunol.177.2.127216818787 [Google Scholar] [CrossRef] [PubMed]
[54]. Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ, HMG-1 as a mediator of acute lung inflammationJ Immunol 2000 165:2950-54.10.4049/jimmunol.165.6.295010975801 [Google Scholar] [CrossRef] [PubMed]
[55]. Lotze MT, Tracey KJ, High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenalNat Rev Immunol 2005 5:331-42.10.1038/nri159415803152 [Google Scholar] [CrossRef] [PubMed]
[56]. Cusick MF, Libbey JE, Fujinami RS, Molecular mimicry as a mechanism of autoimmune diseaseClin Rev Allergy Immunol 2012 42(1):102-11.10.1007/s12016-011-8294-722095454 [Google Scholar] [CrossRef] [PubMed]
[57]. Egerton M, Scollay R, Shortman K, Kinetics of mature T-cell development in the thymusProc Natl Acad Sci USA 1990 87(7):2579-82.10.1073/pnas.87.7.25792138780 [Google Scholar] [CrossRef] [PubMed]
[58]. Starr TK, Jameson SC, Hogquist KA, Positive and negative selection of T cellsAnnu Rev Immunol 2003 21:13910.1146/annurev.immunol.21.120601.14110712414722 [Google Scholar] [CrossRef] [PubMed]
[59]. Takahama Y, Journey through the thymus: stromal guides for T-cell development and selectionNat Rev Immunol 2006 6(2):12710.1038/nri178116491137 [Google Scholar] [CrossRef] [PubMed]
[60]. Powell AM, Black MM, Epitope spreading: protection from pathogens, but propagation of autoimmunity?Clin Exp Dermatol 2001 26:427-33.10.1046/j.1365-2230.2001.00852.x11488833 [Google Scholar] [CrossRef] [PubMed]
[61]. Bonsor DA, Grishkovskaya I, Dodson EJ, Kleanthous C, Molecular mimicry enables competitive recruitment by a natively disordered proteinJ Am Chem Soc 2007 129:4800-07.10.1021/ja070153n17375930 [Google Scholar] [CrossRef] [PubMed]
[62]. Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, A functional and structural basis for TCR cross-reactivity in multiple sclerosisNat Immunol 2002 3(10):940-43.10.1038/ni83512244309 [Google Scholar] [CrossRef] [PubMed]
[63]. Wucherpfennig KW, Strominger JL, Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic proteinCell 1995 80:695-705.10.1016/0092-8674(95)90348-8 [Google Scholar] [CrossRef]
[64]. Evavold BD, Sloan-Lancaster J, Wilson KJ, Rothbard JB, Allen PM, Specific T cell recognition of minimally homologous peptides: evidence for multiple endogenous ligandsImmunity 1995 2:655-63.10.1016/1074-7613(95)90010-1 [Google Scholar] [CrossRef]
[65]. Libby JE, MC Coyy LL, Fujinami RS, Molecular mimicry in multiple sclerosisInt Rev Neurobiol 2007 79:127-47.10.1016/S0074-7742(07)79006-2 [Google Scholar] [CrossRef]
[66]. Ang CW, Jacobs BC, Laman JD, The Guillain-Barré syndrome: a true case of molecular mimicryTrends Immunol 2004 25:61-66.10.1016/j.it.2003.12.00415102364 [Google Scholar] [CrossRef] [PubMed]
[67]. Guilherme L, Ramasawmy R, Kalil J, Rheumatic fever and rheumatic heart disease: genetics and pathogenesisScandinavian Journal of Immunology 2007 66:199-207.10.1111/j.1365-3083.2007.01974.x17635797 [Google Scholar] [CrossRef] [PubMed]
[68]. Fujinami RS, Oldstone MB, Molecular mimicry as a mechanism for virus-induced autoimmunityImmunol Res 1989 8:310.1007/BF029185522647867 [Google Scholar] [CrossRef] [PubMed]
[69]. Benoist C, Mathis D, Autoimmunity provoked by infection: how good is the case for T cell epitope mimicry? Nat Immunol 2001 2:797-801.10.1038/ni0901-79711526389 [Google Scholar] [CrossRef] [PubMed]
[70]. Boyman O, Bystander activation of CD41 T cellsEur J Immunol 2010 40:936-39.10.1002/eji.20104046620309907 [Google Scholar] [CrossRef] [PubMed]
[71]. Tough DF, Zhang X, Sprent J, An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD81 T cell turnover in vivo by IL-12, IL-18, and IFN-gammaJ Immunol 2001 166:6007-11.10.4049/jimmunol.166.10.600711342616 [Google Scholar] [CrossRef] [PubMed]
[72]. Eberl G, Brawand P, MacDonald HR, Selective bystander proliferation of memory CD41 and CD81 T cells upon NK T or T cell activationJ Immunol 2000 165:4305-11.10.4049/jimmunol.165.8.430511035065 [Google Scholar] [CrossRef] [PubMed]
[73]. Berard M, Thoug DF, Qualitative differences between naïve and memory T cellsImmunology 2002 106(2):127-38.10.1046/j.1365-2567.2002.01447.x12047742 [Google Scholar] [CrossRef] [PubMed]
[74]. Trentham DE, Townes AS, Kang AH, Cellular sensitivity to collagen in rhematoid arthritisArchivum Immunologiae et Therapiae Experimentalis 2000 8:347-51. [Google Scholar]
[75]. Cornbay C, Gibbons L, Meyhew V, Sloan CS, B cell epitope spreading: Mechanisms and contribution to autoimmune diseasesImmunology Lettres 2015 163:56-68.10.1016/j.imlet.2014.11.00125445494 [Google Scholar] [CrossRef] [PubMed]
[76]. Tuohy VK, Yu M, Yin L, Kawczak JA, Johnson JM, Mathisen PM, The epitope spreading cascade during progression of experimental autoimmune encephalomyelitis and multiple sclerosisImmunol Rev 1998 164:93-100.10.1111/j.1600-065X.1998.tb01211.x9795767 [Google Scholar] [CrossRef] [PubMed]