Central Nervous System (CNS) and its related tumours are very rare in nature and comprise only 1-2% of total malignancies [1,2]. Glioma is a special type of CNS tumour that arises from glial cells. Gliomas consist of about 30% of all CNS and brain tumours, and 80% of total brain malignant tumours [3]. From an immunological standpoint, acute and chronic inflammation plays an important role in the process of carcinogenesis. Acute inflammation persists for a short time and is beneficial for the host, while chronic inflammation persists for a long time and can influence various chronic illnesses, including cancer [4,5]. In the process of inflammation different immune cells accumulate Reactive Oxygen Species (ROS) at the site of injury or damage [5-7]. Inflammatory cells also produce cytokines, chemokines and other metabolites that can precede the production of additional reactive species at the injury site [5,8]. This continual inflammatory and oxidative milieu leads to injury of neighboring healthy stromal and epithelial cells, which over a long period of time may lead to carcinogenesis [5,9]. Various ROS are capable of oxidizing DNA, thereby producing deoxyribose oxidation products such as 8-hydroxy-2-deoxyguanosine (8OHdG). These products cause double-strand and/or single-strand breaks, chromosomal aberrations, cross-linking and sister chromatid exchanges that can lead to carcinogenesis [5,10,11]. Further, ROS and oxidative stress alter the expression of p53, cell proliferation, invasiveness and metastasis in the process of carcinogenesis [5,10].
Prolidase, a manganese dependent cytosolic enzyme, also known as Xaa-Pro dipeptidase and peptidase D (PEPD) is ubiquitous in nature and cleaves dipeptides. It also plays an important role in proline recycling [12,13]. In the case of stomach cancer, increased collagen degradation and prolidase activity has been reported [14]. Oxidative stress and increased prolidase activity provide the signal for angiogenesis and mediate metastasis in cancerous tissue, while prolidase deficiency leads to impaired angiogenesis [15]. It has also been reported that up-regulation of prolidase expression can be targeted for anti-cancer therapy [16]. It has been seen that increased prolidase activity is related to oxidative stress in several cancers, including stomach cancer, breast cancer, colorectal cancer, ovarian cancer, bladder cancer, renal cell carcinoma and others [14,16-20]. Alternatively, decreased prolidase activity has been reported in chronic pancreatitis and pancreatic cancer patients [21].
Thus, inflammation, oxidative stress, and prolidase activity can be associated with the development of malignancy either individually or in combination. There are limited studies in the literature regarding oxidative stress and prolidase activity in the clinical evaluation of glioma. Thus, the present study was aimed to explore the status of prolidase activity and oxidative stress in the tissues and sera of patients with glioma.
Materials and Methods
The present cross-sectional study was conducted in the Department of Biochemistry with the coordination of the Department of Neurosurgery and the Department of Forensic Medicine of the Institute of Medical Sciences (IMS), Banaras Hindu University (BHU), Varanasi, Uttar Pradesh, India for the period of September 2011 to August 2016. This cross-sectional study was ethically approved by the Institutional (IMS, BHU) Human Ethical Committee. Written informed consent form was taken from the every studied subject. In the case of cadaver subjects, written consent form was taken from their relatives.
Patient Selection
Forty six patients with glioma, 20 cadaver and 46 healthy volunteers were considered as Cases, Control-1 and Control-2 respectively. All of them were matched for age and sex criteria. Diagnosis of cases was done by expert clinicians with the use of Computed Tomography (CT) scan, Magnetic Resonance Imaging (MRI), X-ray, serological examinations and pathological findings.
Tissue and Sera Collection
The selection and collection of samples for the study were categorised into following two categories:
Category-1: About 5 mL blood was collected from each Cases, Control-1 and Control-2. Blood was withdrawn by venipuncture method from peripheral vein (from Cases and Control-2), while aortic blood was collected from Control-1 during autopsy. Sera was separated by centrifugation at 3000 rpm for 10 minutes and stored at -80°C. Repeated thawing of the stored samples was avoided.
Category-2: It included tissue biopsies of 46 patients with glioma and tissue autopsy of 20 cadavers. Tissue biopsies and autopsy were taken in phosphate buffer saline. Samples from cadavers (from the Department of Forensic Medicine during the post-mortem) were taken that were not more than 4 hours after death [11]. However, cancerous tissue (glioma tissue) was collected from the Operation Theatre (OT) of the Department of Neurosurgery during the surgeries.
Tissue Homogenisation
Gliomas tissue samples were homogenised in 1:9 ratios of tissue and phosphate buffer saline as weight: volume, with the help of liquid nitrogen crushing. Homogenised glioma tissues were centrifuged at 3000 rpm for 10 minutes. After centrifugation, the supernatant was taken in another tube and the cell debris was discarded. The supernatant was taken for oxidative stress and prolidase activity measurement. The samples were stored at -80°C until used.
Measurement of Prolidase Activity
Sera were six times diluted during enzymatic activation phase. Thus, enzymatic activity was calculated according to following equation [22]:
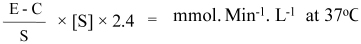
E-Experimental tube absorbance, C-control tube absorbance, S-standard tube absorbance, [S]-concentration of the substrate in mmol/L (94 mmol/L).
Tissue homogenate was 60 times diluted (10 times during tissue homogenisation×6 times during enzymatic activation phase). Thus, results were expressed in per litre of 60-fold diluted tissue homogenate in one minute, we used following equation for tissue prolidase activity representation [23,24]:
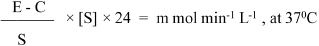
Measurement of Oxidative Stress Parameters
TOS, TAS and OSI of sera as well as tissue homogenate were measured as per previously standardised methods [22,24].
Statistical Analysis
Post-hoc test was used for p-value calculations for the multiples comparing of any two groups (Student-Newman-Keuls test). A p<0.05 considered statistically significant.
Results
Present study comprises total 46 cases of mean age 47.04±12.60 years (29 male and 17 female), 20 Control-1 of 46.75±12.08 years (13 male and 7 female) and 46 Control-2 of 46.87±12.53 years (30 male and 16 female) of matched age and sex (p=0.996) [Table/Fig-1].
Representation of serum SPA, STOS, STAS and SOSI in cases, control-1 and control-2.
| Parameters | Cases Mean±SD (n=46) | Control-1 Mean±SD (n=20) | Control-2 Mean±SD (n=46) | p-value |
|---|
| Age (years) | 47.04±12.60 | 46.75±12.08 | 46.87±12.53 | 0.996 |
| SPA (mmol Minute-1L-1) | 95.46±12.90 | 86.51±5.41 | 83.71±7.70 | <0.001 |
| STOS (mmol H2O2 Eq/L) | 18.91±1.81 | 15.83±2.46 | 11.93±1.80 | <0.001 |
| STAS (μmol Trolox Eq/L) | 1.02±0.14 | 1.36±0.34 | 1.67±0. 34 | <0.001 |
| SOSI (Arbitrary Unit) | 19.23±4.58 | 12.64±4.90 | 7.89±4.00 | <0.001 |
SPA: Sera prolidase activity; STOS: Sera total oxidant status; STAS: Sera total antioxidant status; SOSI: Sera oxidative stress index; n: Numbers of subjects
Sera Prolidase Activity (SPA), Sera Total Oxidant Status (STOS) and Sera Oxidative Stress Index (SOSI) were significantly increased in Cases as compared to Control-1 and Control-2 (all ANOVA p<0.001) [Table/Fig-1]; while Sera Total Antioxidant Status (STAS) was significantly decreased (ANOVA p<0.001) [Table/Fig-1].
Post-hoc test revealed that alteration of SPA, STOS, STAS and SOSI for any two compared groups have significant values (all p<0.01) [Table/Fig-2], except for, the altered values of SPA for Control-1 versus Control-2 that was non-significant (p=0.293) [Table/Fig-2].
Representation of Post-hoc test (Student-Newman-Keuls test’s) p-value for multiple compared groups.
| Variables | Post-hoc test, p-values for multiples compared groups (Student-Newman-Keuls test) |
|---|
| Cases vs. Control-1 | Cases vs. Control-2 | Control-1 vs. Control-2 |
|---|
| SPA | 0.001 | <0.001 | 0.293 |
| STOS | <0.001 | <0.001 | <0.001 |
| STAS | <0.001 | <0.001 | <0.001 |
| SOSI | <0.001 | <0.001 | <0.001 |
SPA: Sera prolidase activity; STOS: Sera total oxidant status; STAS: Sera total antioxidant status; SOSI: Sera oxidative stress index
As sera, Tissue Prolidase Activity (TPA), Tissue Total Oxidant Status (TTOS) and Tissue Oxidative Stress Index (TOSI) were also significantly increased in the glioma tissues of the Cases as compared to Control-1 (all p<0.001) [Table/Fig-3]; while Tissue Total Antioxidant Status (TTAS) was significantly decreased (p<0.001) [Table/Fig-3].
Representation of glioma tissue, TPA, TTOS, TTAS and TOSI in cases and control-1.
| Parameters | Cases Mean±SD (n=46) | Control-1 Mean±SD (n=20) | p-value |
|---|
| TPA (mmol Minute-1L-1) | 398.53±68.23 | 174.09±60.12 | <0.001 |
| TTOS (mmol H2O2 Eq/L) | 22.44±2.79 | 18.74±1.24 | <0.001 |
| TTAS (μmol Trolox Eq/L) | 9.00±1.19 | 12.13±1.09 | <0.001 |
| TOSI (Arbitrary Unit) | 2.57±0.80 | 1.56±0.24 | <0.001 |
TPA: Tissue prolidase activity; TTOS: Tissue total oxidant status; TTAS: Tissue total antioxidant status; TOSI: Tissue oxidative stress index; n: Numbers of subjects
Discussion
Glioma is one of the most common primary brain tumours of the CNS. Inflammation, inflammatory processes, and inflammatory mediators play an important role in the pathogenesis of glioma [25]. Approximately one-third of the tumour load is composed of activated microglia or macrophages. Genetic alterations in glioma can lead to the alteration in expression of various inflammatory genes that lead to staffing of inflammatory cells [25]. On the whole, the tumour microenvironment is largely composed of inflammatory mediators including chemokines, cytokines, ROS, Reactive Nitrogen Species (RNS), NF-κB and COX-2 which can generate a cellular environment favorable for glioma promotion [25]. Thus, there are a number of molecular events that define the molecular connection between inflammation and oxidative stress in the development of glioma [25].
An 8-hydroxy-2-deoxyguanosine (8-OHdG), a modified DNA base produced by means of oxidation of deoxyguanosine, is regarded as the marker of oxidative DNA damage [11,26]. Tuzgen S et al., reported that glioma tissue is exposed to higher oxidative DNA damage (higher concentrations of 8-hydroxy-2-deoxyguanosine; 8-OH-dG) as compared to control tissue; while total antioxidant status decreases in glioma tissue [11]. Another study revealed that different antioxidants like ascorbic acid, α-tocopherol and albumin are decreased in the glioma patients [27]. However, oxidant markers such as γ-GTP, ferritin, coenzyme Q and uric acid are elevated in the patients with glioma [27]. Besides this, other researchers showed that different oxidants have therapeutic potential in glioma treatment [27-29]. However, there are limited studies in the literature regarding the clinical evaluation of glioma patients with reference to TOS, OSI and TAS status in both tissue and sera samples. In the present study, it has been observed that the oxidative stress markers such as TOS and OSI increases while TAS decreases in the patients with glioma as compared to healthy controls, which is well accepted by previous literature. The value of sera TOS and OSI were significantly increased in glioma either than sera of healthy controls or cadaver sera or both [Table/Fig-1,2]; while sera TAS was decreased [Table/Fig-1,2]. The values of these oxidative stress markers (TAS, TOS and OSI) in the case of tissue of glioma followed same patterns; that are TTOS and TOSI status significantly increased, and TTAS status significantly decreased in the patients with glioma as compared to cadaver controls [Table/Fig-3]. When the values of sera oxidative stress markers were compared between cadaver and healthy volunteers then it was seen that STOS and SOSI increased; while STAS was decreased in cadavers than healthy controls [Table/Fig-2]. It means cadaver’s sera had increased oxidative stress as compared to healthy controls.
Prolidase, a member of Matrix Metalloproteinases (MMPs), is an enzyme involved in release of carboxy-terminal proline and hydroxyproline from oligopeptides and also involved in collagen turnover which is related to cell growth [12,30]. The main function of prolidase in human body is recycling of proline and collagen degradation from glycyl-L-proline for collagen re-synthesis. It acts as a rate limiting factor for the regulation of collagen biosynthesis [30,31].
The human body contains collagen as the major Extracellular Matrix (ECM) component in different tissues. An ECM protein such as collagen interacts with cells which regulates cellular gene expression, growth and has potential for tumourigenicity and invasiveness [32]. Cancer is characterised by invasiveness and breakdown of tissue organisation. Breakdown of ECM proteins promotes tumour progression. MMPs secretion is the vital event with progression and metastasis of cancer that is responsible for breakdown of ECM. The tumour cell expresses collagenases that initiate the degradation of collagen and the final step of collagen degradation is mediated by prolidase, all this lead to breaks in ECM as well as tissue barriers that favours metastasis [32]. Thus, prolidase activity has been associated with different events of cancer. It has been reported that prolidase activity increased in a number of cancers like stomach cancer, bladder cancer, renal cell carcinoma, breast cancer, colorectal cancer and ovarian cancer [14,16,20], while some studies have reported decreased prolidase activity in chronic pancreatitis and pancreatic cancer [21]. To the best of our knowledge, there are no more studies in the literature regarding prolidase activity in patients with glioma except a study by Gönüllü E et al., [33]. Gönüllü E et al., carried out the study on the sera of 25 glioma patients and reported that sera prolidase activity was significantly decreased as compared to healthy individuals. On contrary, the present study indicated that sera prolidase activity was significantly increased in the patients with glioma as compared to healthy controls and cadaver controls (ANOVA p<0.001) [Table/Fig-1,2]. Along with this it has been also observed that tissue prolidase activity was significantly increased in the patients with glioma as compared to cadaver controls (p<0.001) [Table/Fig-3]. According to the literature, increased prolidase activity provide signal for angiogenesis and mediates metastasis in cancerous tissue, while prolidase deficiency leads to impaired angiogenesis [31]. Karna E et al., reported that up-regulation of prolidase expression can be targeted for anti-cancer therapy [16]. As indicated by the literature, MMPs (prolidase) are related to different events of cancer such as angiogenesis, invasiveness and metastasis [16,31,32]. Thus, it seems that increased tissue as well as sera prolidase activity in the patients with glioma might be associated with its pathogenesis.
Conclusion
The study concluded that prolidase activity and oxidative stress markers such as TOS and OSI were significantly elevated in the glioma tissues as well as in the sera of patients with glioma as compared to healthy and cadaver controls; while the status of TAS (an oxidative stress marker) was significantly decreased. Previous studies revealed that the elevated oxidative stress and prolidase activity are associated with increased signal for angiogenesis and mediates metastasis in cancerous tissue. Thus, it seems that elevated prolidase activity and oxidative stress may be associated with the pathogenesis of glioma.
SPA: Sera prolidase activity; STOS: Sera total oxidant status; STAS: Sera total antioxidant status; SOSI: Sera oxidative stress index; n: Numbers of subjects
SPA: Sera prolidase activity; STOS: Sera total oxidant status; STAS: Sera total antioxidant status; SOSI: Sera oxidative stress index
TPA: Tissue prolidase activity; TTOS: Tissue total oxidant status; TTAS: Tissue total antioxidant status; TOSI: Tissue oxidative stress index; n: Numbers of subjects