Acrylic resin material is the most popular and widely used denture base material for over 60 years and it is still not the ideal one. The acrylic resin has many shortcomings such as water absorption, dimensional instability, presence of residual monomer, weak impact strength, colour instability, surface porosities and solubility [1].
Surface porosities are a complex phenomenon that originates from several factors. It may be caused by improper mixing of acrylic resin which may contain air bubbles; polymerization shrinkage due to free monomer remains, inadequate packing of the mold or inadequate compression on the flask [2-4].
Solubility of acrylic resin results from leaching out of free monomer and water soluble additives into the oral fluids. The solubility of denture base acrylic resins may have hazardous reactions on the oral mucosa [5].
A study was conducted that water sorption of the acrylic resin may affect durability of acrylic resin denture. Saliva may interact with the polymer chains causing salvation or reversible rupture of weak interchain bonds, irreversible disruption of the polymer matrix and plasticization of the denture [6].
The Development of polymer products resulted in alternative materials to acrylic resin which solves many of its problems. It may include polyamides (nylon plastics), acetal resins (polyoxymethylene based materials), epoxy resins, polystyrene, and polycarbonate resins; that are suited for thermoplastic processing. These new materials have advantages of minimum mouth preparation needed for the removable prosthesis; they are supplied in tooth colour and gingival colour material which guarantee better matching with natural teeth and gingiva, also it can be used in thin sections without fear of fracture which improve patient tolerance and comfort [7,8].
Thermoplastic acrylic was introduced into flexible dental material. It has adequate tensile and flexural strength. It is easy to adjust, handle and polish. The material is available in tooth and gingival colours, providing excellent aesthetics [9].
Surface Porosities and solubility are undesirable characteristics of PMMA that significantly weaken a denture base resin and promote staining, harboring of organisms such as Candida Albicans and bond failures between the artificial tooth and denture base resin. It can result in high internal stresses and vulnerability to distortion and warpage of denture base thereby compromising its physical, aesthetic and hygienic properties [10-12].
The present study emphasized that, the denture base resins will undergo solubility and surface porosities; but the heat cured acrylic resin is more affected than the thermoplastic denture base resin.
The aim of this in vitro study was to evaluate and compare surface porosities and solubility between thermoplastic flexible and conventional heat cured acrylic resin denture base material.
Materials and Methods
The present in vitro experimental study was carried out in the laboratory of the Prosthodontics Department, Faculty of Dental Medicine Al Azhar University, Cairo, Egypt from Jan 2017 to Jan 2018.
Materials
Vertex Thermosens Rigid injection type denture base material (Vertex dental bvj,v. oldenbarneveltin 62 3705Hj zeist The Netherlands) and heat cured acrylic resin (Vertex dental bvj,v. oldenbarneveltin 623705Hj zeist The Netherlands) were used in this study. Manufacturers’ instructions were followed during fabrication of the specimens. Forty samples; (n=20/group) were prepared for each group. Group 1 included flexible poly amide and Group 2 included conventional heat cured acrylic resin. According to the type of test, the samples were distributed in the groups. (Ntest=10/group).
Methods of Sample Fabrication
-Sample fabrication: 40 disc specimens were fabricated from wax pattern with dimensions (20±1 mm in diameter x 0.5 mm in thickness) according to ISO standard number 20795-1:2013 [13].
-Investment of the wax pattern in the mold was done using investing plaster in conventional flask and bodies of wax patterns were submerged in plaster in each flask and the pour was allowed to set for 1 hour.
For wax elimination; the flask containing mold was placed in boiling water for 5 minutes to soften the wax, then the lid were separated and the wax was eliminated, each half of the flask was flushed thrice with application of hot household detergent solution (2 tea spoonful of Nirma powder in 1 liter water) followed by rinsing in boiling water.
-Mixing of polymer and monomer was done according to manufactures’ recommendations, the necessary quantity of monomer was taken into a mixing jar and powder (heat cured acrylic resin (Vertex dental bvj,v. oldenbarneveltin 623705Hj zeist The Netherlands) was added to it, under slow continuous vibration until the layer of liquid disappears. The material was thoroughly mixed with a clean spatula and the mixing jar lid was closed.
-Packing of heat cured acrylic resin specimens was done following the manufactures’ instructions. Heat cured acrylic resin dough was prepared, packed into the flask and the flask was placed in the bench press and was closed.
-Injection of polyamide flexible denture base: According to manufacturer’s instructions poly amide flexible denture base material was injected in the plaster mold using injection machine.
-Curing of specimens: the curing process occurred in conventional curing device at 74°C for 1.5 hours and then the temperature of the water bath was increased to boiling temp for additional one hour. The specimens were removed from the mold and then finished and polished using grit sand paper. [Table/Fig-1] shows disc of specimens’ of thermoplastic polyamide nylon and (PMMA).
Finished disk prepared test specimens.
- Specimens were divided into two groups including 20 specimens in each group
Solubility Measurement
Solubility was measured by registering the desorption of the samples that gained water after 4 weeks. Desorption was done by keeping the specimens in a firmly closed desiccator containing distilled water at 37°C in a thermostatically controlled incubator containing silica gel. The samples were weighed weekly till 6th week when constant weights were attained. The weight after 4th week of keeping the specimens in a firmly closed desiccator containing distilled water was used to calculate the water solubility percentage which represents the amount of leached material from the samples. An electronic balance device with four digit precision was used to measure the specimens [Table/Fig-2].
An electronic balance device with four digit precision.

The specimens were measured according to the following equations:
Water solubility = weight before immersion (dry weight) - weight after 4 weeks of desiccation/Surface area (cm2).
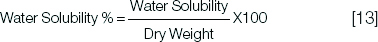
The solubility percentage was calculated at 1 day, 1 week, 2 weeks and one month.
Porosity Measurement
The method used in the present study was supported by Tager A, who reported that the porosity of a sorbate is estimated quantitatively by total pore volume (W0) [14]. Specimens were dried in a containers containing silica gel desiccant. They were weighed daily by an electronic analytical balance (Sartorius, Sartorius AG, Gottingen, Germany) capable of measuring upto 0.0001 g until a constant mass was reached, indicating a state of equilibrium. Weights of specimens were measured after one day, week, 2 weeks and month, respectively.
With dried samples, two weights were made: one with the specimen in air and the other with the specimen immediately immersed in distilled water. Afterwards, the specimen groups were stored in distilled water at 37°C in a thermostatically controlled incubator (PA.3A, Advanced Technology, Egy). These were then weighed at regular intervals until a constant mass indicating a state of water saturation was reached. The weights of specimens equalized after 1 week, 2 weeks, and 4 weeks.
After these periods, the specimens were removed from the water, excess water was removed by blotting with filter paper, and the specimens were weighed. Again, two weights were made: one in air and the other with the specimen immediately immersed in distilled water. After the mass registers of the specimens dried and after absorption or desorption of distilled water, the porosity calculations were made using the following equations [14].


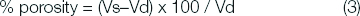
Where, Vd= dried specimen volume; md = mass of dried specimen in air; m’d= mass of dried specimen in water; ρw= density of water; Vs= volume of the specimen saturated by water; ms= mass of saturated specimen in air; and m’s= mass of saturated specimen in water.
In Equations (1) and (2), the volumes were determined using ρw= 1000 Kg/m3. Having solved these equations, the porosity could be calculated by the volume of saturated specimen minus the dried specimen volume divided by the dried specimen, and multiplied by 100 to produce total percent porosity value for each specimen (Equation 3).
Statistical Analysis
The data was collected and statistically analysed using ANOVA test for solubility and porosity. A paired sample t-test and independent t-test at the significance level of (α = 0.05) were used for comparison between two groups regarding different tests.
Results
Solubility
Solubility (%) results recorded for both flexible and conventional groups as function of time are summarized in [Table/Fig-3].
Solubility results (Mean±SD) for flexible and conventional groups as function of time.
| Variables | Storage time | StatisticsANOVAtest |
|---|
| One day | One week | Two weeks | One month | p-value |
|---|
| Flexible | -1.7251±0.4 | -1.54007±0.5 | -2.89617±0.6 | -2.55645±0.8 | 0.0633ns |
| Conventional | -1.57258±0.5 | -1.9716±0.8 | -1.91865±0.7 | -1.65958±0.4 | 0.0814ns |
| p-valueindependent t-test | 0.1559 ns | 0.1032 ns | 0.4302 ns | 0.6987 ns | |
*; significant (p<0.05) ns; non-significant (p>0.05)
The solubility% for flexible group ranged between minimum value (-2.89617±0.6) after two weeks, intermediate values after one month and one day (-2.55645±0.8% and -1.7251±0.4% respectively) and maximum value was (-1.54007±0.5%) after one week. This change in solubility% by time for flexible group was statistically non-significant as indicated by ANOVA test (p-value = 0.0633 >0.05).
The solubility% for conventional group ranged between minimum value (-1.9716±0.8%) after one week, intermediate values after two weeks and one month (-1.91865±0.7% and -1.65958±0.4% respectively) and maximum value (-1.57258±0.5%) after one day. This change in solubility% by time for conventional group was statistically non-significant as indicated by ANOVA test (p-value = 0.0814 >0.05).
Flexible vs. conventional; it was noted that difference between flexible and conventional groups was statistically non-significant at all storage time as indicated by t-test [Table/Fig-3].
Descriptive statistics showing mean values, Standard Deviations (SD) and 95% Confidence Intervals (CI) limits (lower and upper) for total solubility (%) recorded for flexible and conventional groups summarized in [Table/Fig-4].
Comparison of total solubility% results (Mean values±SD) for flexible and conventional groups.
| Variables | Mean±SD | Confidence intervals |
|---|
| Lower | Upper |
|---|
| Denture base material | Flexible | -2.179±0.9 | -2.632 | -1.727 |
| Conventional | -1.781±0.7 | -2.114 | -1.447 |
| paired sample t-test | t-value | 1.2 |
| P-value | 0.2848ns |
ns; non-significant (p>0.05)
The solubility% for flexible group ranged between minimum value (-4.208%) and maximum value was (-0.957%) with total mean±SD values were (-2.179±0.9%).
The solubility% for conventional group ranged between minimum value (-3.003%) and maximum value (-0.897%) with mean±SD values were (-1.781±0.7%).
The difference between flexible and conventional groups was statistically non-significant as indicated by t-test (t = 1.2, p-value = 0.2848 > 0.05).
Porosity
Porosity (%) results recorded for both flexible and conventional groups as function of time are summarized in [Table/Fig-5].
Porosity results (Mean±SD) for flexible and conventional groups as function of time.
| Variables | Storage time | Statistics |
|---|
| One day | One week | Two weeks | One month | p-value ANOVA test |
|---|
| Flexible | 17.26013±1.8 | 11.56236±2.2 | 6.658823±1.5 | 10.54151±2.7 | 0.0005* |
| Conventional | 12.21179±1.6 | 8.94163±0.74 | 2.300097±1.1 | 6.479163±1.2 | <0.0001* |
| p-valueindependent t-test | 0.0106* | 0.0803 ns | 0.006* | 0.0442* | |
*; significant (p<0.05) ns; non-significant (p>0.05)
The porosity% for flexible group ranged between minimum value (6.658823±1.5%) after two weeks, intermediate values after one month and one week (10.54151±2.7% and 11.56236±2.2% respectively) and maximum value (17.26013±1.8%) after one day. With p-value =0.0005.
This change in porosity% by time for flexible group was statistically significant as indicated by ANOVA test (p-value = 0.0005< 0.05)./
The porosity% for conventional group ranged between minimum value (2.300097±1.1%) after two weeks, intermediate values after one month and one week (6.479163±1.2% and 8.94163±0.74% respectively) and maximum value (12.21179±1.6%) after one day, with p-value < 0.0001.
This change in porosity% by time for flexible group was statistically significant as indicated by ANOVA test (p-value = <0.0001< 0.05).
The porosity% for flexible vs. conventional
Flexible group recorded statistically significant higher porosity% than Conventional group after one day with p-value (0.0106*), after two weeks with p-value (0.006*) and after one month with p-value (0.0442*); but after one week storage the difference was non-significant asp-value (0.0803 ns) as indicated by t-test.
Comparison of total porosity% results (Mean values±SD) for flexible and conventional groups
Descriptive statistics showing mean values, SD and 95% CI limits (lower and upper) for total porosity (%) recorded for flexible and conventional groups summarized in [Table/Fig-6].
Comparison of total porosity % results (Mean values±SD) for flexible and conventional groups.
| Variables | Mean±SD | Confidence intervals |
|---|
| Lower | Upper |
|---|
| Denture base material | Flexible | 11.506±4.6 | 9.34 | 13.67 |
| Conventional | 7.483±4.03 | 5.59 | 9.37 |
| paired sample t-test | t-value | 2.9 |
| p-value | 0.006* |
*; significant (p<0.05)
The porosity % for flexible group ranged between minimum value (6.658823%) and maximum value (17.26013%) with total mean±SD values were (11.506±4.6%). The porosity% for conventional group ranged between minimum value (2.300097%) and maximum value (12.21179%) with mean±SD values were (7.483±4.03%).
The difference between flexible and conventional groups was statistically significant as indicated by t-test (t = 2.9, p-value = 0.006< 0.05).
Discussion
Solubility and porosities are implicated properties of denture resins thereby subjecting the material to internal stresses which causes dimensional instability, that may result in crack formation and eventually fracture of the denture. Water sorption and release depends on the degree of hydrophobicity and porosity of the resin material. From a clinical point of view, when denture base resin materials are immersed in an aqueous solution such as water, saliva, nasal secretion or cleansing agents they are vulnerable to water sorption and solubility [5,13,15].
Heat polymerized acrylic resins generally consist of a powder (co-polymer) and liquid (monomer) [9]. Takabayashi Y has suggested that some of their contents like plasticizer materials (Dibutyl phthalate), initiator (benzoyl peroxide) and some unreacted monomer are soluble and have direct relation to the weight of the denture as evidenced by solubility test [16]. Within the first few days of water storage, the largest amount of residual monomer is usually leached from the resins. Consequently, it might be difficult to estimate the effect of residual monomer released from resins with the ADA standardized solubility test [17,18].
In the present study, water solubility was measured by registering the desorption of samples that gained water after 4 weeks then weigh ed weekly till 6 weeks when constant weights were attained. This method is in agreement with Shah J et al., the weight after 4W was used to calculate the water solubility percentage which represents the amount of leached material from the samples. The maximum ADA standard values of water solubility for denture base materials are 1.6 μg/mm2 [13].
According to Shah J et al., the results of solubility values between PMMA and flexible (thermoplastic polyamide nylon) resin were statistically significant as the Heat cure PMMA samples showed more solubility values than flexible resin [13]. These results are in disagreement with the present study as the result showed non-significant difference between two materials in solubility test.
Over a period of time, denture resins primarily absorb water slowly due to the polar properties of the resin molecules. The absorbed water can act as a plasticizer which soften the resin and reduce it’s strength. The resin polarity may control the extent and rate of water uptake into polymer networks, it was dictated by the concentration of polar sites available to form hydrogen bonds with water and network topology [13,19]. The water pools among the polymers of the acrylic resin by diffusion, and pulls them apart, slightly expanding the resin causing micropores. Bacteria and fungi may lodge in micropores that form in the resin, which enhance plaque formation and adversely affect proper cleaning of the prosthesis [20,21].
Furthermore, polymerization of resins is an exothermic reaction, and the increase in temperature can cause the boiling of the reactive monomer, which leads to the formation of bubbles in the resin [20]. The porosity of resins has been described by Oliveira RE et al., as the resin showed the lowest percentage of porosity, corresponding to 0.24% of the resin surface [21]. In comparison the present study showed that, flexible resin recorded statistically significant higher porosity than conventional resin after one day with p-value (0.0106*), after two weeks with p-value (0.006*) and after one month with p-value (0.0442*); but after one week storage the difference was non-significant as p-value (0.0803 ns).
There are different methods to measure the porosity, either when surface area of 1cm2 was delimited in the center of each specimen and observed under 40x magnification, the number of pores per area was calculated for each specimen and an average value was delivered for each group. Others measured the porosity by weighing the specimen in air and in water and used equations to calculate percent mean porosity for the specimen [22-25].
In the present study, the porosity of resins was determined using Archimedes’ principle. After resin processing, the specimens were dried in a containers containing silica gel desiccant, then weighed daily using an electronic analytical balance until a constant mass was reached, weights of specimens equalized after day, 1 week, 2 weeks and 1 month respectively. This method comes in agreement with Tager A who reported that the porosity of a sorbet is estimated quantitatively by total pore volume [14].
Limitation
This in vitro study has a limitation that different beverages and food intake with masticatory action can affect the propagation of solubility and porosity. In the oral cavity, moisture contamination and thermal variation facilitate the absorption of water [26,27].
Another limitation was that the cleansing mode and storage of the dentures can also affect the integrity of denture material. Therefore, future clinical studies required to evaluate the effect of oral environment on solubility and surface integrity of the used denture base material.
Conclusion
There is no difference in solubility between both denture base resin materials; there is statistically significant difference in porosity between both denture base resin materials. Flexible denture bases should be limited to replacement of teeth in the aesthetic zone, with restricted mouth opening, severe soft and hard tissue undercuts or allergy to acrylic or metal.
Conflict of interest: The authors declare that there is no conflict of interest for the publication of this research.