Staining Ability of Turmeric-Persica Solution and Turmeric-GC-MI Plus Paste on Remnant Adhesive Resin after Bracket Debonding
Hossein Aghili1, Mahmood Golzarian2, Soghra Yassaei3, Zahra Ebrahiminik4, Zahra Moradi5
1 Associate Professor, Department of Orthodontics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
2 Assistant Professor, Department of Agriculture, Ferdowsi University, Mashhad, Razavi Khorasan, Iran.
3 Professor, Department of Orthodontics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
4 Orthodontist, Dental Clinic of Emam Reza Hospital, Mashhad University of Medical Sciences, Mashhhad, Iran.
5 Associate Professor, Department of Orthodontics, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Zahra Moradi, Postgraduate Student, Department of Orthodontics, Fajr BLVD, Yaz, Yazd, Iran.
E-mail: zahramoradi442@gmail.com
Introduction
Cleanup, the tooth-coloured adhesive used in orthodontics is time-consuming which may cause the iatrogenic enamel damage. Various techniques have been proposed to reduce the operator-sensitivity of the composite detection procedure. Applications of ink dye and 0.2 brilliant green solutions and articulating paper are some examples.
Aim
To compare the staining effects of Turmeric-Persica solution and Turmeric-GC-MI Plus paste on remnant adhesive on teeth after bracket debonding.
Materials and Methods
Forty metal brackets were bonded to 40 caries-free extracted premolars. After debonding, teeth were randomly divided into two groups of 20 each. Teeth in Group 1 (G1) were immersed in Turmeric-Persica solution while in Group 2 (G2) Turmeric-GC-MI Plus paste was applied to the buccal surface of the teeth. After rinsing and drying, all the specimens were photographed under standard situations and analysed by MATLAB software to evaluate the colour differences between enamel and adhesive. Collected data were statistically analysed by SPSS version 16.0 and compared by Mann-Whitney U test. The significance level was considered to be 0.05.
Results
The mean cross-border yellowness was significantly higher in G2, turmeric-GC-MI Plus paste, than G1, turmeric-persica solution. (with a p-value: 0.005).
Conclusion
Turmeric powder mixed in GC-MI Plus paste could be used as a disclosing material for adhesive resin applied to tooth surface.
Adhesive, Colour, Orthodontics, Turmeric powder
Introduction
The first step of fixed orthodontic therapy is bonding the brackets to the enamel surface of the teeth by a resin adhesive which should be removed at the completion of treatment while debonding. Commonly, some resin adhesive might remain on the teeth surface after bracket debonding which need to be thoroughly removed in order to prevent the accumulation of plaque and may act as a mechanical irritation to the gingiva, that could increase the incidence of periodontal problems [1,2]. Also, discolouration of adhesive due to the poor adhesive removal would become a source of patient dissatisfaction.
Since the adhesive used in orthodontics is tooth-coloured, the process of adhesive cleanup could be problematic and time-consuming [3]. Determining the precise boundaries of adhesive and enamel is difficult which may cause the iatrogenic enamel damage [4]. In routine practice, usually this process is done by visual inspection under unit light and tactile sensation. However, this greatly depend on the experience and skills of the practitioner [5].
Various methods have been introduced in an effort to reduce the operator-sensitivity of the adhesive detection procedure. Applications of ink dye and 0.2 brilliant green solutions are some examples [6]. Rachala MR et al., introduced a method of contrasting the residual adhesive with articulating paper [7]. However, they are toxic and non FDA approved and are used only in forensic dental identification [8,9]. Also, using ultraviolet light and quantitative light-induced fluorescence have been proposed to be useful in detecting the adhesive resin, based on the different fluorescence properties between natural teeth and adhesive resin [10-14] which is not truly practical because the fluorescence emission is better visualised in a dark environment [12]. In addition, most of these techniques require expensive equipment [13,14]. Because of these limitations developing a simple practical method using FDA approved agents which could disclose the composite is extremely in demand. Abdallah MN et al., reported that the solution of turmeric in combination with methylene blue could efficiently stain the composite [6].
The aim of this study was to provide a media for staining protocol of Abdallah MN et al., in order to make it more applicable in clinical situations. Persica solution and GC-MI Plus paste were chosen to be used as the media for turmeric. Persica is a FDA approved herbal mouthwash, commonly prescribed for fixed orthodontic patients [15]. GC-MI Plus paste is a toothpaste containing 900 ppm fluoride which is also extremely recommended for patients during or immediately after orthodontic treatment [16]. Although the active substance for dying the composite (turmeric), was similar in both study groups, using a vehicle to simplify applying turmeric was assumed to have possible effects on colouring properties of turmeric. Therefore, this study compared the staining effects of turmeric-persica solution and turmeric-GC-MI Plus paste.
Materials and Methods
This study was approved by the Committee of Ethics of the School of Dentistry at the Shahid Sadoughi University of Yazd, Iran.
Case Selection
This in-vitro study was conducted in Yazd dental lab during September 2017 to October 2017. Sample size was determined to be 40 extracted teeth based on similar studies [17,18]. To be included in this study, teeth must be caries-free (extracted due to orthodontic treatment) and with intact buccal surface. Teeth with any evidence of crack, fracture or discolouration on buccal surface were excluded from the study. All the teeth (premolars) were mounted in dental stone blocks. Forty metal brackets were bonded to the central part of buccal surface of the teeth using Transbond XT adhesive (3M unitek, USA). Teeth were stored in distilled water for at least 24 hours allowing for polymerisation to be completed.
Staining Process
Brackets were debonded and the samples were divided into two groups of 20. Each group was treated with one of the following materials for 30 seconds:
Group 1: Turmeric-Persica solution: Based on Abdallah MN et al., study [6], 0.1 gram of turmeric powder (ParsiTeb, Shiraz, Iran) was dissolved in 100 ml ethanol. Then, 100 ml persica (Pursina pharmaceutical Co, Iran) was added. Teeth were immersed in the prepared solution for 30 seconds.
Group 2: Turmeric-GC-MI Plus paste: Turmeric solution was prepared same as the first group then it was dissolved in 40 gram GC-MI Plus paste (GC Co., Ltd., Japan). This creamy agent was rubbed to the buccal surface of the samples by an applicator for 30 seconds.
After 30 seconds, teeth were thoroughly rinsed and dried with an oil free air spray.
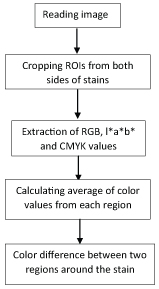
After the staining, to examine the colour difference between the stained adhesive remaining and adjacent enamel surface some colour features were extracted from the images of the teeth. The images were taken with a Nikon CoolPix (Nikon Inc, Japan) with the resolution of 3264 x 2448 pixels. To take images under standard and repeatable situation, a box with a hole for camera lens and a step at the other side for resting the samples was created. Therefore an equal and stable distance between camera and teeth was maintained. The extracted colour features included R, G, B, L*, a*, b* and C, M, Y, K values. RGB colour space is defined by the three chromaticity components of the red, green, and blue, L*a*b* colour space consists of luminance or light intensity (L*), intensity of red to blue (a*) and intensity of green to blue (b*). CMYK colour space stands for Cyan, magenta, yellow and black. For colour feature extraction, a Region Of Interest (ROI) with 15*15 pixels was considered. The regions of interest from images were cropped, which were two regions inside the resin adhesive region and two around on the tooth [Table/Fig-1]. Colour differences between adhesive and enamel was evaluated both at right and left to increase the accuracy. The process of extracting colour features from the images was conducted in Matlab (ver 2015a, Mathworksinc, US). The flowchart of this process is shown in [Table/Fig-2].
Regions of interest: Two regions inside the resin adhesive region and two around on the tooth.

Flowchart of colour analysis process.
To capture the best composite-enamel contrast and eliminate the effects of environment light, teeth were imaged twice under the same conditions except for the flash light that was on or off. The flash was on for all samples and once off for all cases again, then the best images were selected. Since each image was compared with itself (enamel surface was compared to adhesive surface) via MATLAB software, flash light would not be a confounding factor.
Analysing all ten factors, the mean differences of yellowness (1-green) was found to be the most relevant factor in showing the adhesive-enamel contrast; therefore it became the fundament of this analysis.
Statistical Analysis
The collected data was entered to a computer and statistically analysed using SPSS software version 16. Since the variables of this study were continuous and not normally distributed Mann-Whitney U test was chosen to compare the results of study groups.
Results
In all of the studied samples, the contrast was found at the enamel-composite border. The mean cross-border yellowness was higher in group 2 (turmeric-GC-MI Plus paste) than group1 (turmeric-persica solution). Mann-whitney test showed that this difference between groups was statistically significant (p<0.05). [Table/Fig-3] illustrates the descriptive values of yellowness in study groups.
Comparison of yellowness (obtained by MATLAB) in study groups.
| Group | Mean±SD | Maximum | Minimum | Result* |
|---|
| 1 | 0.10±0.03 | 0.15 | 0.04 | p-value< 0.05 |
| 2 | 0.26±0.12 | 0.65 | 0.19 | |
Discussion
Several studies showed that colour stability of adhesive resins could be affected by foods and drinks such as red wine, coffee and tea [20,21]. Cörekçi B et al., reported that using 6 different orthodontic adhesive resin, composite discolourations occurred when the teeth were immersed in tea, cola, coffee, red wine and yogurt [22]. Turmeric and red wine caused the highest discolouration in all adhesive resins tested in a previous work [23]. Abdallah MN et al., reported that a solution of 0.1 gram turmeric powder in 100 ml ethanol could efficiently colour the composite so that the practitioner could easily distinguish the boundaries of composite from the enamel surface [6]. However, application of an alcohol solution in clinical practice might not be healthy nor easy for the patient [24]. Therefore, this study was designed to evaluate the staining ability effects of two different media for turmeric in an attempt to make this potential disclosing agent more practical.
Results of this study showed that application of turmeric- GC-MI Plus paste statistically made more enamel-adhesive contrast than turmeric-persica solution. This could be explained by the fact that in G1, teeth were immersed in the disclosing agent while in G2 disclosing paste was applied on a controllable area of enamel. Obviously immersion would expose the enamel to the disclosing agent much more than paste application. Also, the difference in the vehicle used can be the reason for more uptake of stain in the paste. This requires more chemical analysing investigations to fully clarify the exact reason.
During orthodontic treatment usually the gingival tissue is inflamed and white spots might form on enamel surface [25]. Literature suggested prescribing antibacterial mouth rinse and low-fluoride toothpastes during and/or after the orthodontic treatments particularly for patients with poor oral hygiene [26,27]. Therefore, it is an additional advantage to use persica or GC-MI Plus paste as a media for disclosing agent.
Limitation
The main limitation of this study was in-vitro examination of the samples while the conditions of oral environment particularly the biofilm on teeth surfaces might affect the amount of colour uptake. Also, taking photographs for analysing the difference of colour between enamel and adhesive would not be easy to perform in daily clinical use. One more limitation was that thermocycling was not done in the present study to simulate the oral environment. Also not using the artificial saliva is the shortcoming of this research. Therefore, future studies must be done in-vivo examining variety of the concentrations to find a disclosing agent which could easily be detected with bare eyes. It is also recommended that further clinical studies should be done for investigation of the effects of this suggested disclosing material on different orthodontic adhesives.
Conclusion
GC-MI Plus paste is an appropriate media for turmeric in order to dye the adhesive as a disclosing material.
[1]. Retief D, Denys FR, Finishing of enamel surfaces after debonding of orthodontic attachmentsThe Angle orthodontist 1979 49(1):1-10. [Google Scholar]
[2]. Sukontapatipark W, El-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA, Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy studyThe European Journal of Orthodontics 2001 23(5):475-84.10.1093/ejo/23.5.47511668867 [Google Scholar] [CrossRef] [PubMed]
[3]. Hosein I, Sherriff M, Ireland AJ, Enamel loss during bonding, debonding, and cleanup with use of a self-etching primerAmerican Journal of Orthodontics and Dentofacial Orthopedics 2004 126(6):717-24.10.1016/j.ajodo.2003.10.03215592221 [Google Scholar] [CrossRef] [PubMed]
[4]. David VA, Staley RN, Bigelow HF, Jakobsen JR, Remnant amount and cleanup for 3 adhesives after debracketingAmerican Journal of Orthodontics and Dentofacial Orthopedics 2002 121(3):291-96.10.1067/mod.2002.12100811941343 [Google Scholar] [CrossRef] [PubMed]
[5]. Meller C, Klein C, Fluorescence properties of commercial composite resin restorative materials in dentistryDental materials journal 2012 31(6):916-23.10.4012/dmj.2012-07923207195 [Google Scholar] [CrossRef] [PubMed]
[6]. Abdallah MN, Light N, Amin WM, Retrouvey JM, Cerruti M, Tamimi F, Development of a composite resin disclosing agent based on the understanding of tooth staining mechanismsJournal of dentistry 2014 42(6):697-708.10.1016/j.jdent.2014.03.00424632474 [Google Scholar] [CrossRef] [PubMed]
[7]. Rachala MR, Kishore MS, Vamsilatha K, Staining adhesive remnants for easy removalJ Clin Orthod 2013 47(11):672 [Google Scholar]
[8]. Benthaus S, DuChesne A, Brinkmann B, A new technique for the postmortem detection of tooth-coloured dental restorationsInternational journal of legal medicine 1998 111(3):157-59.10.1007/s0041400501389587800 [Google Scholar] [CrossRef] [PubMed]
[9]. Midda M, A disclosing solution for synthetic fillingBritish dental journal 1969 127(11):519 [Google Scholar]
[10]. Clark D, Ruddick R, Post mortem detection of tooth coloured dental restorations by ultra violet radiationActa medicinae legalis et socialis 1985 35(1):278 [Google Scholar]
[11]. Hermanson AS, Bush MA, Miller RG, Bush PJ, Ultraviolet illumination as an adjunctive aid in dental inspectionJournal of forensic sciences 2008 53(2):408-11.10.1111/j.1556-4029.2008.00657.x18366574 [Google Scholar] [CrossRef] [PubMed]
[12]. Tani K, Watari F, Uo M, Morita M, Discrimination between composite resin and teeth using fluorescence propertiesDental materials journal 2003 22(4):569-80.10.4012/dmj.22.56915005233 [Google Scholar] [CrossRef] [PubMed]
[13]. Carson DO, Orihara Y, Sorbie JL, Pounder DJ, Detection of white restorative dental materials using an alternative light sourceForensic science international 1997 88(2):163-68.10.1016/S0379-0738(97)00115-1 [Google Scholar] [CrossRef]
[14]. Pretty IA, Smith PW, Edgar WM, Higham SM, The use of quantitative light-induced fluorescence (QLF) to identify composite restorations in forensic examinationsJ forensic Sci 2002 47(4):831-36.10.1520/JFS15468J12136993 [Google Scholar] [CrossRef] [PubMed]
[15]. Sofrata A, Brito F, Al-Otaibi M, Gustafsson A, Short term clinical effect of active and inactive Salvadora persica miswak on dental plaque and gingivitisJournal of Ethnopharmacology 2011 137(3):1130-34.10.1016/j.jep.2011.07.03421798329 [Google Scholar] [CrossRef] [PubMed]
[16]. Huang GJ, Roloff-Chiang B, Mills BE, Shalchi S, Spiekerman C, Korpak AM, Effectiveness of MI Paste Plus and PreviDent fluoride varnish for treatment of white spot lesions: a randomized controlled trialAmerican Journal of Orthodontics and Dentofacial Orthopedics 2013 143(1):31-41.10.1016/j.ajodo.2012.09.00723273358 [Google Scholar] [CrossRef] [PubMed]
[17]. Malhotra N, Shenoy RP, Acharya S, Shenoy R, Mayya S, Effect of Three Indigenous Food Stains on Resin-Based, Microhybrid-, and NanocompositesJournal of Esthetic and Restorative Dentistry 2011 23(4):250-7.10.1111/j.1708-8240.2011.00431.x21806757 [Google Scholar] [CrossRef] [PubMed]
[18]. Ren YF, Feng L, Serban D, Malmstrom HS, Effects of common beverage colourants on colour stability of dental composite resins: the utility of a thermocycling stain challenge model in vitroJ Dent 2012 40(Suppl 1):e48-56.10.1016/j.jdent.2012.04.01722542498 [Google Scholar] [CrossRef] [PubMed]
[19]. Li H, Lai L, Chen L, Lu C, Cai Q, The prediction in computer colour matching of dentistry based on GA+BP neural networkComput Math Methods Med 2015 2015:81671910.1155/2015/81671925873990 [Google Scholar] [CrossRef] [PubMed]
[20]. Bagheri R, Burrow M, Tyas M, Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materialsJournal of Dentistry 2005 33(5):389-98.10.1016/j.jdent.2004.10.01815833394 [Google Scholar] [CrossRef] [PubMed]
[21]. Scotti R, Mascellani SC, Forniti F, The in vitro colour stability of acrylic resins for provisional restorationsInternational Journal of Prosthodontics 1997 10(2):164-68. [Google Scholar]
[22]. Çörekçi B, Irgın C, Malkoç S, Öztörk B, Effects of staining solutions on the discolouration of orthodontic adhesives: an in-vitro studyAmerican Journal of Orthodontics and Dentofacial Orthopedics 2010 138(6):741-46.10.1016/j.ajodo.2008.12.02921130333 [Google Scholar] [CrossRef] [PubMed]
[23]. Stober T, Gilde H, Lenz P, Colour stability of highly filled composite resin materials for facingsDental Materials 2001 17(1):87-94.10.1016/S0109-5641(00)00065-8 [Google Scholar] [CrossRef]
[24]. McCullough M, Farah C, The role of alcohol in oral carcinogenesis with particular reference to alcohol-containing mouthwashesAustralian Dental Journal 2008 53(4):302-05./10.1111/j.1834-7819.2008.00070.x19133944 [Google Scholar] [CrossRef] [PubMed]
[25]. Tufekci E, Dixon JS, Gunsolley J, Lindauer SJ, Prevalence of white spot lesions during orthodontic treatment with fixed appliancesThe Angle orthodontist 2011 81(2):206-10.10.2319/051710-262.121208070 [Google Scholar] [CrossRef] [PubMed]
[26]. Palombini AB, Comparison of Three Fluoride Dentifrice Products in the Prevention of White Spot Lesions in Orthodontic Treatment 2015 The University of Alabama at Birmingham [Google Scholar]
[27]. Bergstrand F, Twetman S, A review on prevention and treatment of post-orthodontic white spot lesions–evidence-based methods and emerging technologiesThe Open Dentistry Journal 2011 5(1):158-62.10.2174/187421060110501015821966335 [Google Scholar] [CrossRef] [PubMed]