Prevalence of Sexually Transmitted Diseases Detected in Cervical Cytology Smears in Urban and Rural Population of Lucknow, Uttar Pradesh, India
Jata Shankar Misra1, Anand Narain Srivastava2, Hem Prabha Gupta3
1 Senior Research Officer (Cytology), Department of Pathology, Era’s Lucknow Medical College and Hospital, Lucknow, Uttar Pradesh, India.
2 Director Research, Department of Pathology, Era’s Lucknow Medical College and Hospital, Lucknow, Uttar Pradesh, India.
3 Professor and Head, Department of Obstetrics and Gynaecology, Era’s Lucknow Medical College and Hospital, Lucknow, Uttar Pradesh, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Anand Narain Srivastava, Director Research, Department of Pathology, Era’s Lucknow Medical College and Hospital, Lucknow-226003, Uttar Pradesh, India.
E-mail: ans4csmmu@gmail.com
Introduction
Sexually Transmitted Diseases (STDs) are very common in the sexually active young Indian women particularly in rural areas because of poor genital hygiene.
Aim
The present study was carried out to compare the prevalence of different STDs in the urban and rural population of Lucknow, Uttar Pradesh.
Materials and Methods
The present prospective observational study was conducted in 2369 rural women attending camps in 126 villages of West Lucknow between May 2013 and March 2017 undergoing cervical cancer screening and in 38,478 urban women attending Gynaecology Outpatient Department (OPD) of KG Medical University, Lucknow, Uttar Pradesh, India, between April 1971 to November 2005 in which the data were collected retrospectively. Statistical analysis was performed with SPSS version 18.0 and the results were subjected to chi-square test.
Results
The incidence of Candida albicans was higher in rural women (4.7%) as against 1.1% seen in urban cohorts. The trend was reverse with Trichomonas vaginalis, the incidence being high (2.9%) in the Urban women than 1.0% in the rural group. The viral STDs were rarely seen in both the groups ranging from 0.1% to 0.6%. Association of Squamous Intraepithelial Lesions (SILs) of the cervix with non-viral STDs was more pronounced in rural women than in their urban counterparts while this was very high with viral STDs in both the groups. All the STDs except HSV were commonly seen in younger sexually active women between 21-30 years. Leucorrhoea was commonly associated with all STDs in both groups but the incidence was almost double in rural women.
Conclusion
All the STDs examined were commonly seen in younger women between 21-30 years mostly with symptoms of leucorrhoea. Hence, cytological screening is felt mandatory in such women to rule out any STD infection.
Candida albicans, Cervical neoplasia, Human papillomavirus, Trichomonal infection
Introduction
Many types of STDs infest human female genital tract; some are non viral like Candida albicans (fungus), Trichomonas vaginalis (protozoan) and Chlamydia trachomatis (bacteria), while others are viral like HPV and HSV. While some of them produce symptomatic changes in the genital tract like vaginal discharge, others like viral STDs are commonly associated with pre-malignant and cancerous changes in the cervix. In recent years, HPV has been widely implicated in the process of cervical carcinogenesis and in fact, it is now well established that HPV is a causal factor in the development of cervical intraepithelial neoplasia and invasive carcinoma [1-4]. HSV has been commonly found under pre-malignant and malignant conditions of cervix and synergism of action of both these viruses has been suggested to play role in the malignant transformation of the cervical epithelium [5]. Since we have sufficient data of cervical cytology in the urban and rural population of Lucknow, it was thought worthwhile to compare the incidence of different STDs in these two cohorts and different pre-disposing factors related to the cervical carcinogenesis. The comparative data obtained from the extensive analysis of the findings in the two cohorts are presented in this study which has been scarcely attempted.
Materials and Methods
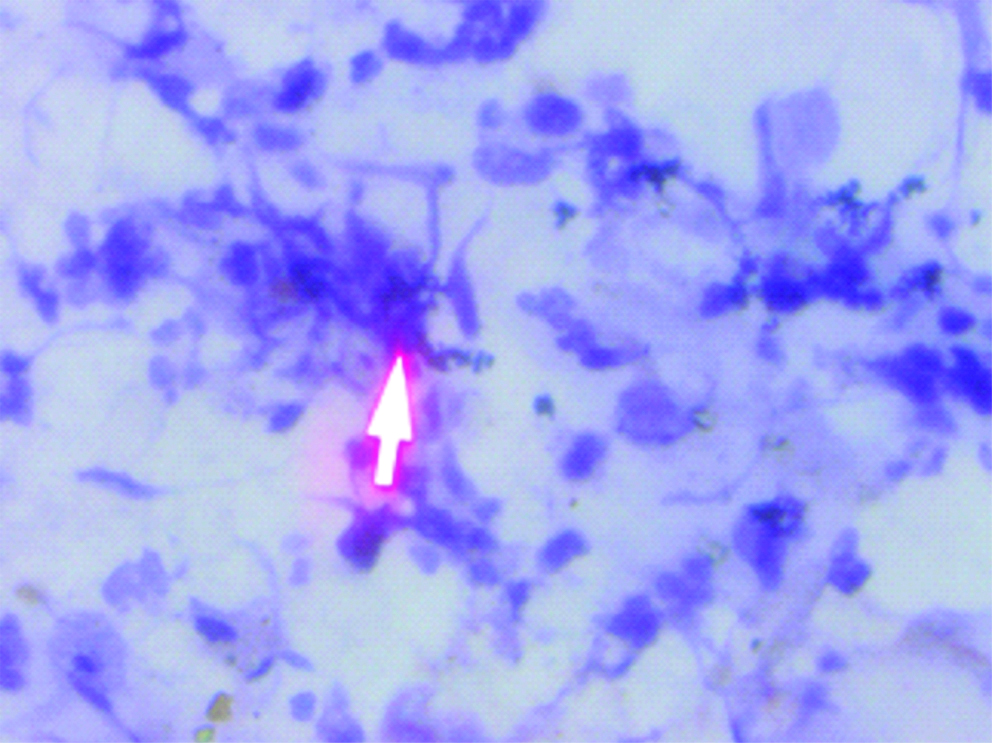
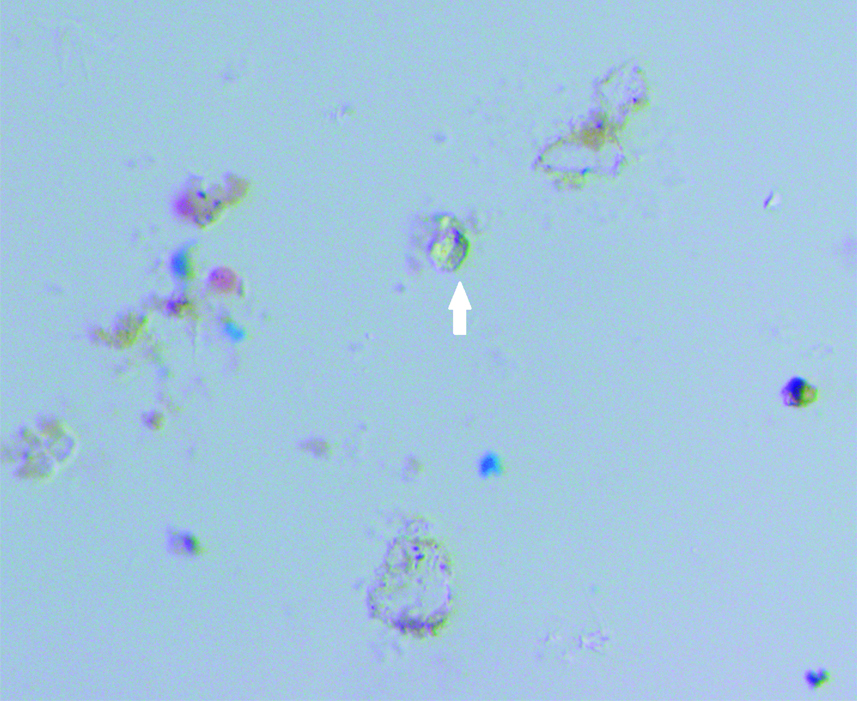
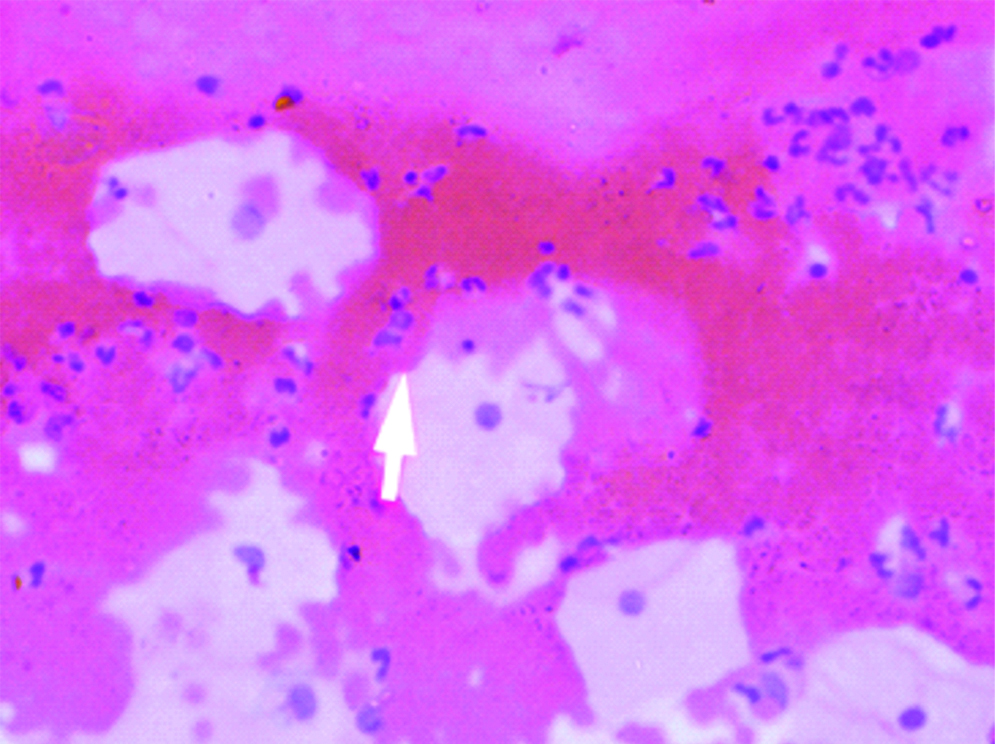
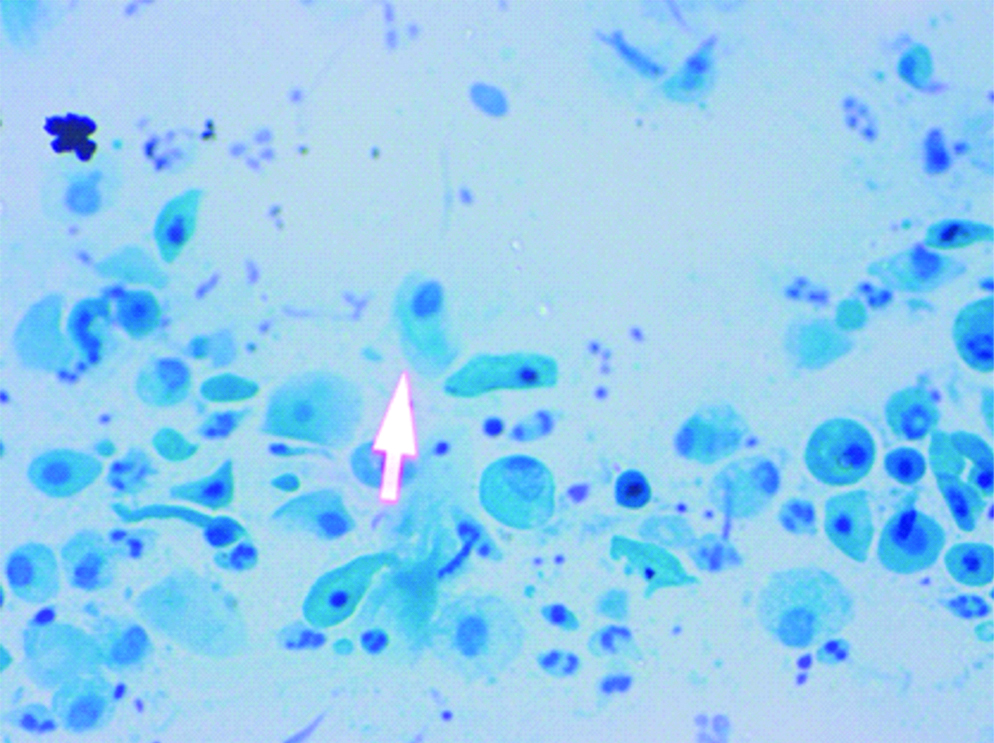
A prospective observational study was conducted in which screening of cervical smear of women for early detection of carcinoma cervix in the urban and rural population of Lucknow was done. Ethical clearance was obtained from the Ethical Committee of the Era’s Lucknow Medical College and Hospital, Lucknow before starting the rural cancer screening program. Informed consent forms were also obtained from these women attending the camp during the rural cervical cancer screening. Cervical cytology smears were screened for detection of the STDs in the urban population of Lucknow and rural population of West Lucknow. The urban screening was opportunistic in women attending OPD of Queen Mary’s Hospital, of KG Medical University, Lucknow, the rural screening was planned in women counselled for attending the camps. The number of urban women screened was 38,478 between a span of 35 years (April 1971-November 2005) while 2369 rural women were cytologically examined who attended the camps organised between May 2013 to March 2017 in the 126 villages of West Lucknow. Only women between the ages of 21-60 years were included in the study and those who have undergone the hysterectomy were not included. In each case, a scrape smear was taken from the squamocolumnar junction of cervix prior to each speculum examination and fixed in absolute alcohol. The cervical smears were stained according to Papanicaclaou’s technique and cytopathological changes observed were graded according to the Bethesda system of reporting cervical smears of 1993 [6]. In the urban screening, prior to this, WHO classification of 1973 was followed [7]. There is no significant difference between these two classifications and cytology reports made according to the WHO classification between 1971 to 1992 have been rearranged according to the Bethesda system of reporting. The cervical smears were also evaluated for individual presence of Candida albicans in the form of hyphae or spores [Table/Fig-1] and Trichomonas vaginalis in the trophozoite form [Table/Fig-2] and also for any cytomorphological changes produced by the viral STDs-HPV (koilocytosis) [Table/Fig-3] and HSV (ground glass appearance of nuclei) [Table/Fig-4]. The women attending the camps were given the cytology reports and treatment according to the type of STD detected in the cytology smears.
Cervical smear: Candida albicans-hyphae and spores (Pap, 40X).
Cervical smear: Trichomonas vaginalis-trophozoites (Pap, 40X).
Cervical smear: Human papillomavirus: koilocytes (Pap, 40X).
Cervical smear: Herpes simplex virus: ground glass appearance of nuclei (Pap, 40X).
Statistical Analysis
The statistical analysis of the data was carried out by the chi-square test (SPSS version 18.0).
Results
The four types of STDs namely: Candida albicans, Trichomonas vaginalis, Human Papillomavirus (HPV) and Herpes Simplex Virus (HSV) were detected in cytology smears during cervical cancer screening in the rural and urban population of Lucknow. The women screened in both the cohorts ranged from 21-60 years. The incidence of four STDs microorganisms in the two cohorts of 2369 rural and 38,478 urban women is given in [Table/Fig-5]. The statistical analysis revealed highly significant difference in the incidence of Candida and Trichomonas infection in the urban and rural population (χ2=215.05; p<0.0001 and χ2=30.08; p<0.0001) respectively. However, the difference in the incidence of HPV and HSV was insignificant in the two populations (χ2=0.003; p=0.960 and χ2=1.95; p=0.162 respectively).
Incidence of different STDs in urban and rural population screened.
| STDs | Rural population | Urban population |
|---|
| Number of cases | Incidence | Number of cases | Incidence |
|---|
| Candida albicans | 112 | 4.7% | 440 | 1.1% |
| Trichomonal infection | 24 | 1.0% | 1130 | 2.9% |
| HPV | 12 | 0.5% | 192 | 0.5% |
| HSV | 3 | 0.1% | 108 | 0.28% |
Association of the four STD microorganisms with SIL in the two cohorts are shown in [Table/Fig-6]. The relation with SIL was more pronounced in the rural women being 23.2% with Candida as against 8.4% in urban women and the difference was significant (χ2=13.35; p<0.001). However, in case of trichomonal infection, SIL incidence was almost similar in both the cohorts (16.6% in rural women as against 11.8% in their urban counterparts) and the difference was insignificant (χ2=0.516; p=0.473). The concomitant occurrence of the cytomorphological changes produced by the two viral STDs with SIL was higher in both the cohorts ranging from 33.3% to 75% in rural women and 31.7% to 36.1% in the urban population. However, the incidence of HPV infection was significantly different between the two populations (χ2=9.36; p=0.0002). The incidence of HSV infection in the two cohorts showed insignificant difference (χ2=0.01; p=0.921).
Association of SIL with different STDs.
| STDs | Rural population | Urban population |
|---|
| Number of cases | Incidence of SIL | Number of cases | Incidence of SIL |
|---|
| Candida albicans | 112 | 26 (23.2%) | 440 | 37 (8.4%) |
| Trichomonal infection | 24 | 4 (16.6%) | 1130 | 134 (11.8%) |
| HPV | 12 | 9 (75%) | 192 | 61 (31.7%) |
| HSV | 3 | 1 (33.3%) | 108 | 39 (36.1%) |
The relation of STDs with age had also been analysed and shown in [Table/Fig-7]. The incidence of the four STD microorganisms showed no significant difference in the two cohorts in the different age groups.
Relation of STDs with age.
| Age group | Candida albicans | Trichomonas vaginalis | Human papillomavirus | Herpes simplex |
|---|
| Rural (112) | Urban (440) | Rural (24) | Urban (1130) | Rural (12) | Urban (192) | Rural (3) | Urban (108) |
|---|
| <20 years | 5 (4.4%) | 16 (3.6%) | - | 43 (3.8%) | - | 3 (1.5%) | - | - |
| 21-30 years | 46 (41.1%) | 219 (49.7%) | 16 (66.6%) | 529 (46.8%) | 4 (33.3%) | 62 (32.2%) | - | 3 (2.7%) |
| 31-40 years | 48 (42.8%) | 168 (38.1%) | 6 (25.0%) | 474 (41.9%) | 4 (33.3%) | 65 (33.8%) | 1 (33.3%) | 41 (37.9%) |
| Beyond 40 years | 13 (11.6%) | 37 (8.4%) | 2 (8.3%) | 84 (7.4%) | 4 (33.3%) | 62 (32.2%) | 2 (66.6%) | 64 (59.2%) |
Association of the four STD microorganisms with gynaecological symptoms has been investigated and shown in [Table/Fig-8]. Leucorrhoea was the most common symptom seen in the women of both cohorts who were infected with both viral and non-viral STDs. However, incidence of Candida and Trichomonas was almost double in the rural group than in their urban counterparts (68.7% as against 37.7% with Candida and 79.1% as against 42.03% with Trichomonas). The difference in the incidence of Candida and Trichomonas was significant among the two groups (χ2=34.86; p <0.001) and (χ2=13.23; p=0.003) respectively. The trend was reverse with viral STDs, the incidence being higher in the urban than in the rural women but the difference was statistically insignificant with both HPV and HSV in the leucorrhea patients. All the STDs were also seen with different menstrual disorders and bleeding but the frequency was not high. The only significant difference was seen with Trichomonal infection (χ2=33.97; p=0.001).
Relation of the STDs with gynaecological symptoms.
| Symptoms | Candida albicans | Trichomonas vaginalis | Human papillomavirus | Herpes simplex |
|---|
| Rural (112) | Urban (440) | Rural (24) | Urban (1130) | Rural (12) | Urban (192) | Rural (3) | Urban (108) |
|---|
| Leucorrhea | 77 (68.7%) | 166 (37.7%) | 19 (79.1%) | 475 (42.03%) | 3 (25%) | 34 (17.7%) | - | 17 (15.7%) |
| Menstrual disorders | 3 (2.6%) | 2 (0.4%) | 3 (12.5%) | 12 (1.06%) | 1 (8.3%) | 16 (8.3%) | - | 17 (15.7%) |
| Contact bleeding | - | 12 (2.7%) | - | 18 (1.5%) | - | - | - | 8 (7.4%) |
| Post menopausal bleeding | - | - | - | - | - | 6 (3.1%) | - | 4 (3.7%) |
The relation between four STD microorganisms and clinical lesions of cervix is shown in [Table/Fig-9]. All the STDs were commonly seen with erosion and hypertrophic cervix. The viral STDs were commonly seen in women with bleed on touch cervices. The statistically high value was seen only with Candida in the two cohorts in the case of hypertrophied cervix and cervicitis (χ2=6.34; p<0.001 and χ2=14.92; p<0.001 respectively).
Relationship of the STDs with clinical lesions of cervix.
| Clinical lesions of cervix | Candida albicans | Trichomonas vaginalis | Human papillomavirus | Herpes simplex |
|---|
| Rural (112) | Urban (440) | Rural (24) | Urban (1130) | Rural (12) | Urban (192) | Rural (3) | Urban (108) |
|---|
| Erosion cervix | 17 (15.1%) | 114 (25.9%) | 3 (12.5%) | 302 (26.7%) | - | 46 (23.9%) | - | 24 (22.2%) |
| Hypertrophied cervix | 1 (0.9%) | 65 (14.7%) | 2 (8.3%) | 213 (18.8%) | - | 28 (14.5%) | - | 11 (10.1%) |
| Cervix bleeds on touch | 2 (1.8%) | 2 (0.4%) | 1 (4.1%) | 26 (2.3%) | 1 (8.3%) | 9 (4.6%) | - | 11 (10.1%) |
| Cervicitis | 1 (0.9%) | 58 (13.1%) | - | 152 (13.4%) | 1 (8.3%) | 14 (7.2%) | - | 24 (22.2%) |
Discussion
In the present study, the incidence of Candida albicans was higher than that of Trichomonas vaginalis in the rural population while in the urban women; trichomonal infection was more common than Candida. Results were in concordance with Misra JS et al., [8,9]. Some investigators like, Srivastava M et al., Nikumbh DB et al., and Arora BB et al., on the contrary, had reported high incidence of Trichomonas vaginalis in rural women than Candida [10-12] However, the incidence of Candida and Trichomonas have been found very low by the present investigators ranging from 1.1% in the urban population to 4.7% in the rural women in the case of Candida and 1.0% in the rural as against 2.9% in the urban women for Trichomonas vaginalis. Sethwala ND, et al., have reported a high incidence of 10.3% of candidal infection among female sex workers in Surat [13]. Fule SR et al., have also found 12.1% candidal infection in the rural women of Karnataka [14]. Barouti E et al., have also reported 11% of candidal infection in Iran [15]. A very high incidence of Candida has been reported in Turkey by Guduco N et al., [16]. However, Bukhari MH et al., have also found a low incidence of Candida (3.0%) in Pakistan like in the present study [17].
As regards trichomonal infection, its high incidence (8.5%) was reported by Madhivanan P et al., in Karnataka [18] Abreu AL et al., have also found Trichomonas vaginalis the most prevalent STD (11.6%) and its co-infection with high risk-HPV increased the risk of Atypical Squamous Changes of Undetermined Significance (ASCUS) cytology and High grade Squamous Intraepithelial Lesions (HSILs) of cervix [19]. On the contrary, Verteramo R et al., have not found any correlation between TV and HPV infection [20]. Mendez C et al., have also seen 31% of trichomonal infection in the cervical smears in their series of women studied [21] However, Leuv SL et al., have seen 9.1% of TV infection in their cases studied [22].
The incidence of SIL was higher with candidal infection (23.2%) in rural population than in urban population (8.4%). It appears that candidal infection remains undetected and untreated for long time in the rural women due to lack of medical amenities which causes inflammation, persistence of which for long duration may lead to SIL changes in the cervix. In fact, Dalie F et al., have opined that Candida causes protein degradation and enhances antigenic response leading to the mucosal injuries and endogenous invasion [23]. They have seen that the extent of damage incurred is also determined by the inherent properties of epithelial cells like state of maturity. Mayer P et al., have found that in the endogenous fungal infection, tissue debris and accumulation of free radicals enhances virulence of the organism and increases the susceptibility of the host [24]. Hence, the association of Candida with cervical lesions may be related to its inflammatory effects. Kone ES et al., have found association of Candida with HPV infection in 57.8% of cases showing a significant relationship between the two STDs [25].
The trichomonal infection association with SIL was almost similar in rural and urban population. As reported earlier, Abreue AL et al., have also found correlation between Trichomonas vaginalis and high risk-HPV increases the risk of ascus cytology and HSIL [19]. Kone ES et al., have also reported HPV infection in 5% of the Trichomonas vaginalis infested cases [25].
Both HPV and HSV infection have been found frequently associated with SIL. This association was more pronounced in rural population. A high occurrence of SIL with viral STDs shows their strong affinity with cervical neoplasia. Epidemiological and molecular investigations have unequivocally shown that high grade HPV infection is the causal factor initiating the progressive transformation that leads to the CIN into the cervical neoplasia. Wohlmaster D et al., have found HPV-DNA in 20.7% of cytology samples they have studied and have seen good correlation between cytological changes like ascus, LSIL and HSIL and HPV infection (28.0%) [26].
Various studies of HSV-2 on the viral load, serological status and co-infection with HPV have been performed [27,28]. However, only a few studies have explored the correlation between HSV-DNA and squamous cell carcinoma using PCR based methods with variable results. Abreu AL et al., have found all cases of co-infection with HPV and HSV-2 occurring specially with high risk-HPV. The co-infection of high risk-HPV with HSV-2 contributed a ten times higher increased risk of ascus cytology but no increased risk to HSIL was observed. These results support the ‘hit and run’ mechanism which states that HSV-2 participates in some initial phases of cervical carcinogenesis but does not require its retention. Therefore, the HSV-2 is not detected consistently in all cervical biopsies of cervical lesions/squamous cell carcinoma which suggest that HSV may be necessary for the initial transformation of cells but not for the squmous cell carcinoma progression [29].
Vaginal discharge was the most common symptom associated with different types of STDs. The non-viral STDs displayed high percentage in leucorrhoea cases and this was more pronounced in the rural women. It appears that as most of the rural women are illiterate due to lack of knowledge regarding personal genital hygiene; they harbour vaginal infection which remains persisted for long time due to not being detected and treated. Wohlmaster D et al., have seen that 54% of women complaining of vaginal discharge were positive for STD [26]. Other authors like Fule SR et al., and Masand DL et al., have reported a low incidence of Candida and Trichomonas in vaginal discharge cases among rural women [14,30]. Khan SA et al., have also reported a low incidence of Trichomonas infection with vaginal discharge in Pakistan [31].
All the STDs except HSV were common in young sexually active women between 21-30 years of age while HSV was mostly seen after 40 years of age in the postmenopausal women. Barouti E et al., have also found that the overall prevalence of STDs was greater among reproductive age [15]. Leuv SL et al., have also found 26.3 mean age of trichomonal infection [22]. Madhivanan et al., have found a relatively high burden of T. vaginalis among young reproductive aged women and have suggested that since the Trichomonas infection increases the risk of HIV transmission and is associated with adverse pregnancy outcome, there is a need for increased screening and treatment of this easily curable STD infection in India [18].
Limitation
The viral STDs have been seen to play possible role in the aetiology of cervical cancer predominantly in its pre-invasive phase. Hence the cytological detection of STDs should be made mandatory in a cytological screening program for checking the persistence of associated symptoms and onset of the SIL. However, the detection of all these STDs made in the cytology smears should be confirmed by the proper test. This was not possible in the present study both in the urban and rural setup as the women mostly did not turn up for follow-up.
Conclusion
This comparative study revealed a high incidence of Candida albicans in rural women while Trichomonas vaginilis was found more common in the urban women. Both these STDs were commonly seen in the sexually active younger women between 21-30 years of age and were mostly associated with persistent vaginal discharge. Hence, detection of these STDs is necessary to cure vaginal discharge and other associated side effects. In the rural women, illiteracy and poverty have been the main reason of poor genital hygiene causing persistent vaginal discharge.
[1]. Lorinez AT, Hybrid capture method in detection of HPV-DNA in clinical specimens PapillomavirusRep 1996 7:01-51. [Google Scholar]
[2]. Zur Hausen H, Molecular pathogenesis of cancer of cervix and its causation by specific humanpapillomavirus typeCurr Top Microbiol Immunol 1994 186:131-56.10.1007/978-3-642-78487-3_88205839 [Google Scholar] [CrossRef] [PubMed]
[3]. Bosch FX, Manos MM, Muñoz N, Prevalence of humanpapillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study GroupJ Natl Cancer Inst 1995 87(11):796-802.10.1093/jnci/87.11.7967791229 [Google Scholar] [CrossRef] [PubMed]
[4]. Walboomers JM, Jacobs MV, Monos MM, Bosch FX, Kummer JA, Shah KV, HPV is a necessary cause of invasive cervical cancer worldwideJ Pathol 1999 189:12-19.10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F [Google Scholar] [CrossRef]
[5]. Balbi C, Grazia F, Piscitialli V, Retrospective study of cervical Papillomavirus lesions: early Herpes simplex virus proteins as marker of risk for progressionMinova Ginecologica 1998 48(3):175-79. [Google Scholar]
[6]. Riotton G, Christopherson WM. Cytology of female genital tract. International histological classification of tumors. No. 8. World Health Organization Geneva. 1973 [Google Scholar]
[7]. National Cancer Institute Workshop 1991Bethesda system of reporting vaginal: cervical cytological diagnosisActa Cytol 1993 37:115-24. [Google Scholar]
[8]. Misra JS, Srivastava AN, Gupta HP, Results of cervical cancer screening in the rural population of Lucknow West, India, through a camp approachActa Cytol 2018 62(4):273-78.10.1159/00048907829898440 [Google Scholar] [CrossRef] [PubMed]
[9]. Misra JS, Das V, Singh U, Sexually transmitted disease and cytopathology of cervixJ Cytol 2004 21(2):68-72. [Google Scholar]
[10]. Srivastava M, Srivastava OP, Jaiswal SS, Pattern of cervical smear cytology in rural Medical CollegePravara Med Rev 2011 3:04-08. [Google Scholar]
[11]. Nikumbh DB, Nikumbh RD, Dombale VD, Jagtap SV, Desai SB, Cervicovaginal cytology: Clinicopathological and social aspect of cervical cancer screening in rural (Maharashtra) IndiaInt J Health Sci Res 2012 1:125 [Google Scholar]
[12]. Arora BB, Maheshwari M, Devgan N, Arora DR, Prevalence of trichomonasis, vaginal candidasis, genital herpes, chlamydiasis and actinomycosis among urban and rural women of Haryana, IndiaJ Sex Transm Dis 2014 2014:96381210.1155/2014/96381226316979 [Google Scholar] [CrossRef] [PubMed]
[13]. Sethwala ND, Mulla SA, Kosambiya JK, Dunn VK, Sexually transmitted infections and reproductive tract infections in female sex workersIndian J Pathol Microbiol 2009 52:188-99.10.4103/0377-4929.4891619332911 [Google Scholar] [CrossRef] [PubMed]
[14]. Fule SR, Fule RP, Tankhiwale S, Clinical and laboratory evidence of Trichomonas vaginalis infections among women of reproductive age in rural areaIndian J Med Microbiol 2012 30:314-18.10.4103/0255-0857.9949322885198 [Google Scholar] [CrossRef] [PubMed]
[15]. Barouti E, Farzoneh F, Sene AA, Tajik Z, Jafari B, The pathogenic micro-organisms in Papanicolau vaginal smears and correlation with inflammationJ Family Reprod Health 2013 7:23-27. [Google Scholar]
[16]. Guduco N, Gonene G, Isci H, Yigiter AB, Bassullu N, Dunder I, Clinical importance of detection of Bacterial vaginosis, Trichomonas vaginalis, Candida albicans and Actinomyces in Papanicolau smearsClin Exp Obstet Gynaecol 2012 39:333-36. [Google Scholar]
[17]. Bukhari MH, Majeed M, Qamar S, Niazi S, Syed SZ, Yusuf AW, Clinicopathological study of Papanicolau (Pap) smears for diagnosis of cervical infectionsDiagn Cytopathol 2012 40:35-41.10.1002/dc.2149820949462 [Google Scholar] [CrossRef] [PubMed]
[18]. Madhivanan P, Bartman MP, Pasutti L, Krupp K, Arun A, Reingold AL, Prevalence of Trichomonas vaginalis infection among young reproductive age women in India: Implication for treatment and preventionSex Health 2009 6:331-44.10.1071/SH0903819917204 [Google Scholar] [CrossRef] [PubMed]
[19]. Abreu AL, Malaguti W, Souza RP, Uchimura NS, Ferreira EC, Pereira M, Association of HPV, Neisseria gonorrhae and Chlamydia trichomatis- co-infections on the risk of HSIL of cervical lesionsAmer J Cancer Res 2016 6(6):1371-83. [Google Scholar]
[20]. Verteramo R, Pierangeli A, Manani E, Calzotun E, Bucci M, Osbom J, Human Papillomavirus and genital co-infections in gynaecological atrophitisBMC Infect. Dis 2009 9:1610.1186/1471-2334-9-1619216747 [Google Scholar] [CrossRef] [PubMed]
[21]. Mendez C, Castellsaque S, Renom M, Secartal J, Quinto L, Uuvoreas B, Prevalence and risk factors of sexually transmitted infections and cervical neoplasia in women from rural areas of South MozambiqueInf Dis Obstet Gynaecol 2010 2410:60931510.1155/2010/60931520706691 [Google Scholar] [CrossRef] [PubMed]
[22]. Leuv SL, Kmda KA, Bemotein KT, Pajitente JC, Rusaxo AM, Carceres CP, Trihomonas vaginalis infection and associated risk factors in socially marginalized population in Coastal PeruInf Dis Obstet Gynaecol 2009 2009:75243710.1155/2009/75243719584943 [Google Scholar] [CrossRef] [PubMed]
[23]. Dalie F, Watchler B, L’oliver C, Holland G, Bannert G, Wilson D, Cellular interaction of Candida albicans with human oral epithelial cells and enterocytesCell Microviol 2010 12:248-71.10.1111/j.1462-5822.2009.01394.x19863559 [Google Scholar] [CrossRef] [PubMed]
[24]. Mayer P, Wilson G, Huba B, Candida albicans: Pathogenecity mechanismsVirulence 2013 4:119-28.10.4161/viru.2291323302789 [Google Scholar] [CrossRef] [PubMed]
[25]. Kone ES, Balili A, Paparisto PP, Ceka XR, Petreia EP, Vaginal infections of Albanian women infected with HPV and their impact on intraepithelial cervical lesions as evidenced by Pap testJ. Cytol 2017 34(1):16-21.10.4103/0970-9371.19759228182076 [Google Scholar] [CrossRef] [PubMed]
[26]. Wohlmaster D, Vianna DRB, Heifer VE, Gimervas F, Consolan MEL, Barcello RB, Association of human Papillomavirus infection and Chlamydia trachomatis with intraepithelial alterations in cervical samplesMem Inst Oswalw Reode Janso 2016 11(2):18610.1590/0074-0276015033026841046 [Google Scholar] [CrossRef] [PubMed]
[27]. Lazenby GB, Taylor PT, Badman BS, McHaki E, Korte JE, Soper DE, An association between Trichomonas vaginalis and high risk Human Papillomavirus in rural Tanzanian women undergoing cervical cancer screeningClin Ther 2014 36:38410.1016/j.clinthera.2013.11.00924417784 [Google Scholar] [CrossRef] [PubMed]
[28]. Kalyani R, Virus and cervical cancer: Role and implication: A reviewBiomed Res Ther 2015 2:220-30.10.7603/s40730-015-0007-z [Google Scholar] [CrossRef]
[29]. Paba P, Bonifacio D, Di Bonito L, Ombres D, Favall C, Syijanen K, Co-expression of HSV-2 and Chlamydia trachomatis in HPV-positive cervical cancer and cervical intraepithelial neoplasia is associated with aberrations in key intracellular pathwaysIntervirology 2008 51:230-34.10.1159/00015648118812695 [Google Scholar] [CrossRef] [PubMed]
[30]. Masand DL, Patel J, Gupta S, Utility of microbiological profile of symptomatic vaginal discharge in rural women of reproductive age groupJ Clin Diag Res 2015 9:QC04-07.10.7860/JCDR/2015/12161.562325954668 [Google Scholar] [CrossRef] [PubMed]
[31]. Khan SA, Amir F, Altaf S, Tanveer R, Evaluation of common organisms causing vaginal dischargeJ Ayub Med Coll Abbottabad 2009 21:90-93. [Google Scholar]