Proton pump inhibitors are one of the commonly prescribed drugs for peptic ulcer. Commonly prescribed PPIs are omeprazole, rabeprazole, pantoprazole and lansoprazole. PPIs are prescribed for both the approved and off label conditions. US FDA approved PPIs are indicated in peptic ulcer, gastritis, NSAIDs induced peptic ulcer, bleeding peptic ulcer, Gastroesophageal Reflux Disease (GERD) and Zollinger Ellison syndrome [1].
Increase in usage of PPI is due to their availability as Over the Counter Drugs (OTC) by physician’s prescriptions. It has been reported that between 25% to 70% of prescriptions do not have proper indication [2]. These are found to be one of the over utilised drugs due to their effectiveness in suppressing the symptoms [3].
According to National Health and Nutrition Examination Survey results, 7.8% of US adults had used prescription PPIs in the previous 30 days which was less than real prevalence as these drugs were available as OTC drugs [4].
These preparations are used very irregularly by the patients even after the completion of prescribed duration. The adverse effects of PPIs are megaloblastic anaemia, atrophy of gastric mucosa, hyper-gastrinaemia, osteoporosis, hypomagnesemia and infections such as community acquired pneumonia [5] and Clostridium difficile [6] infections.
According to WHO, kidney disease is responsible for nearly 850,000 deaths every year, of which CKD is the 12th leading cause of death and 17th leading cause of disability [7]. The annual incidence of CKD in India is approximately 150-200 per million populations (pmp) [8].
In a review on PPI use and kidney disease, it has been stated that many isolated case reports and case series on development of AKI due to PPI use and has also been described that PPI-induced AIN might cause irreversible inflammation in the renal interstitium, which may have negative long-standing effects on the kidney [11].
A cohort of 10,482 who were self-reported PPI users with eGFR at least 60 mL/ minute/1.73 m2 in Atherosclerosis Risk in Communities (ARIC) study were followed and findings were replicated in patients receiving care in Geisinger Health System was analysed by Lazarus B et al., and the study results revealed that PPIs use was associated with 20-50% higher risk of incident CKD [12].
Arora P et al., conducted two independent retrospective case-control studies with prospective logistic regression analysis of data had shown that among those who developed CKD, 24.4% were found to be on PPIs [13]. A recent retrospective study had shown that 19 (10.86%) out of 175 patient who were on PPIs for at least seven consecutive days was found to have acute kidney injury. Pantoprazole was the most common drug involved (84.21%) and AKI common in the age group above 50 years [14].
From India, only a few case reports and retrospective analysis of kidney disease have been observed in relation to PPIs use. As there were no prospective studies linking the duration of PPIs use with kidney disease, present study was undertaken.
The present study was aimed to assess the renal parameters-serum creatinine and BUN in patients on PPIs and to correlate these parameters with demographic data, duration and type of PPIs use as well as to estimate eGFR and correlate with stage of CKD.
Materials and Methods
A prospective observational study was carried out at Chettinad Hospital and Research Institute (CHRI), Kelambakkam during the period from July to August 2017.
The sample size was calculated to 400, basied on prevalence of PPIs use of nearly 50%. It was difficult to collect data from 200 test and 200 control in three months period for ICMR STS project, hence we conducted a pilot study taking minimum sample size of 60 (test group-30 and Control group-30). Control group subjects who were not taken PPIs for more than one year and these participants were selected from CHRI who were accompanying the patients.
Inclusion criteria: Male and female subjects aged 18 to 60 years, attending to CHRI, patients using any of the PPIs and who were willing to participate in the study and provide written informed consent were included in the study.
Exclusion criteria: Pregnant women and lactating mother, patients with known renal disease, hypertension, diabetes mellitus as well as patients on other antiulcer medications and on drugs that may likely cause renal damage (Aminoglycosides, cephalosporins, vancomycin, anticancer drugs etc.,) were excluded from the study.
Study was initiated after getting Institutional Human Ethics Committee approval (Ref No: proposal no:246/IHEC/5-17). Subjects were selected from outpatient or inpatient department at the study site. Subjects were selected according to inclusion and exclusion criteria. After enrollment written informed consent was obtained from willing subjects before starting the study. Demographic data and medication details of the subjects were collected. About 5 mL of blood sample in the fed state was collected in the morning for assessing serum creatinine and BUN.
Serum creatinine values per se are not used to assess renal function in human beings and assessment of renal function is done by calculating eGFR based on the measured serum creatinine. The eGFR will give the functioning status of kidney whether it is normal or deranged.
eGFR is calculated by the abbreviated MDRD equation:
=186×(Creatinine/88.4)-1.154×(Age)-0.203×(0.742 if female)
Based on eGFR, staging of kidney disease to be done [15] as shown in [Table/Fig-1].
Stage of kidney disease and eGFR.
| S No. | Stage and eGFR value |
|---|
| 1. | Stage 1-eGFR ≥90 mL/minute/1.73 m2 |
| 2. | Stage 2-eGFR between 60-89 mL/minute/1.73 m2 |
| 3. | Stage 3-eGFR between 30-59 mL/minute/1.73 m2 |
| 4. | Stage 4-eGFR between 15-29 mL/minute/1.73 m2 |
| 5. | Stage 5-eGFR decreases to less than 15 mL/minute/1.73 m2 |
Statistical Analysis
Values were expressed as Mean±SD and results were analysed by Student’s unpaired t-test and one-way ANOVA. Correlation of biochemical parameters was done with demographic data, duration of PPIs use and stage of kidney disease. A p-value <0.05 was considered statistically significant.
Results
A total of 60 subjects were recruited according to inclusion and exclusion criteria. Data on demographic details such as age, sex, occupation and education were collected from all subjects and given in [Table/Fig-2].
Demographic details in control and test group.
| Demographic details | Group |
|---|
| Control | Cases |
|---|
| Count | % | Count | % |
|---|
| Age (years) | ≤20 | 12 | 40.00 | 8 | 26.67 |
| 21-30 | 4 | 13.33 | 6 | 20.00 |
| 31-40 | 6 | 20.00 | 3 | 10.00 |
| 41-50 | 4 | 13.33 | 6 | 20.00 |
| 51-60 | 4 | 13.33 | 7 | 23.33 |
| Sex | Male | 20 | 66.67 | 26 | 86.67 |
| Female | 10 | 33.33 | 4 | 13.33 |
| Occupation | Business | 6 | 20.00 | 7 | 24.14 |
| Self employed | 2 | 6.67 | 6 | 20.69 |
| Professional | 5 | 16.67 | 3 | 10.34 |
| Retired | 1 | 3.33 | 1 | 3.45 |
| Student | 14 | 46.67 | 10 | 34.48 |
| Housewife | 2 | 6.67 | 2 | 6.90 |
| Not given | 0 | 0 | 1 | 3.33 |
| Education | Not given | 1 | 3.33 | 1 | 3.33 |
| Primary | 0 | 0 | 3 | 10.34 |
| High School | 7 | 24.14 | 7 | 24.14 |
| Degree | 16 | 55.17 | 17 | 58.62 |
| Postgraduate | 6 | 20.69 | 2 | 6.90 |
Medication details in test group: The test group was prescribed the commonly used PPIs such as pantoprazole, omeprazole and rabeprazole. Among the PPIs, 18 (60%) were on pantoprazole, 6 (20%) on omeprazole and 6 (20%) on rabeprazole. Only 60% of the test group participants were using PPIs regularly for prescribed duration and 40% were using irregularly.
Duration of PPI use: About 13 (43.33%) participants were using PPI between one and three months. 6 (20%), 7 (23.3%) and 4 (13.3%) were using PPI for <1 month, 3-6 months and >6 months respectively.
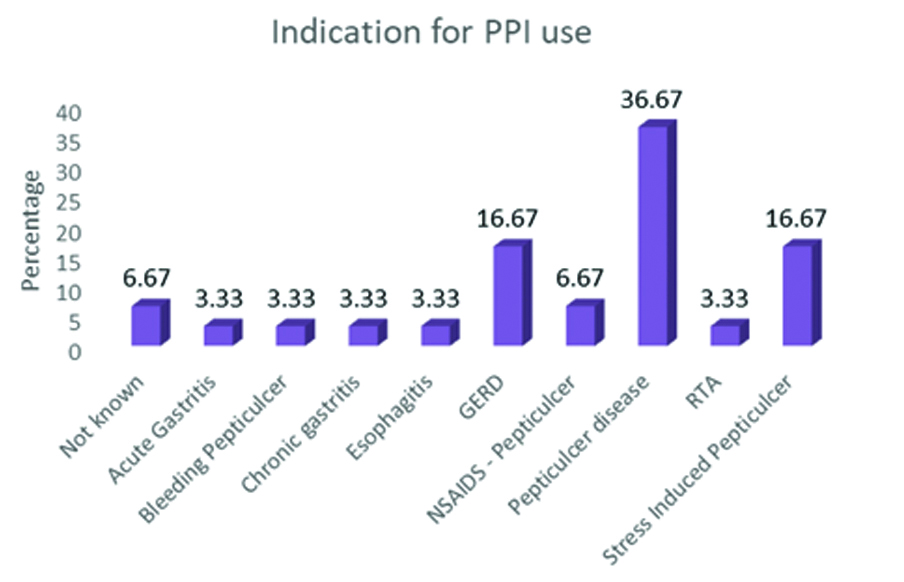
PPI indications: About 11 (36.6%) were using for peptic ulcer disease and 5 (16.6%) for both GERD and stress induced peptic ulcer. Other indication details are given in [Table/Fig-3].
PPI indications in test group.
N=30, values expressed as percentage
Renal parameters: The mean serum creatinine in control group was 0.80 and in test group 1.03 mg/dL. Though the serum creatinine in the test group after PPI therapy was within the normal range, the difference between control and test group was significant (p<0.05) [Table/Fig-4].
Serum creatinine and blood urea nitrogen in control and test group.
| Variables | Group | Independent Samples t-test |
|---|
| Control | Test |
|---|
| Mean | SD | Mean | SD | t-value | p-value |
|---|
| Serum creatinine (mg/dL) | 0.80 | 0.16 | 1.03 | 0.41 | -2.932 | 0.006* |
| BUN (mg/dL) | 10.90 | 2.63 | 12.73 | 5.10 | -1.750 | 0.087 |
*p-value significant
BUN: The mean BUN was 10.90 mg/dL in control and 12.73 mg/dL in test group. The difference was not found to be statistically significant [Table/Fig-4].
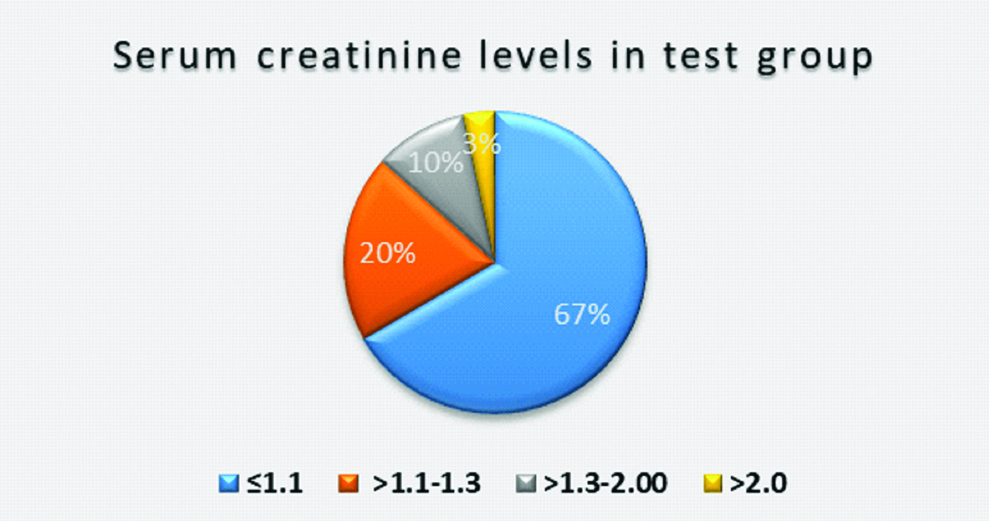
The serum creatinine in the test group was graded in to four for the purpose of the study as <1.1, 1.1-1.3, 1.3 to 2 and >2 mg/dL and percentage of participants is represented in the [Table/Fig-5].
Serum creatinine levels in test group.
N=30 serum creatinine levels expressed in mg/dL
There was no significant difference observed with regularity of use, type of PPI and change in both serum creatinine and BUN [Table/Fig-6] however, significant difference (p<0.05) was observed with duration of PPI and BUN and not with serum creatinine [Table/Fig-7].
Correlation of renal parameters with duration and regularity of use.
| Regularly/irregular | Independent Samples t-test |
|---|
| Regular | Irregular |
|---|
| Mean | SD | Mean | SD | t-value | p-value |
|---|
| Duration | 3.81 | 2.49 | 3.04 | 3.06 | 0.752 | 0.458 |
| Serum creatinine (mg/dL) | 1.02 | 0.32 | 1.05 | 0.54 | -0.216 | 0.831 |
| BUN mg/dL | 12.89 | 6.32 | 12.50 | 2.58 | .201 | 0.842 |
Correlation of renal parameters with duration and type of PPI use.
| Drug | One-way ANOVA |
|---|
| Omeprazole | Pantoprazole | Rabeprazole |
|---|
| Mean | SD | Mean | SD | Mean | SD | F-value | p-value |
|---|
| Duration | 5.42 | 2.54 | 3.31 | 2.74 | 2.17 | 1.94 | 2.524 | 0.099 |
| Serum creatinine (mg/dL) | 1.36 | 0.26 | 0.99 | 0.46 | 0.84 | 0.14 | 2.983 | 0.068 |
| Blood urea nitrogen (mg/dL) | 18.00 | 7.87 | 11.56 | 3.55 | 11.00 | 2.00 | 5.193 | 0.012* |
*p-value significant
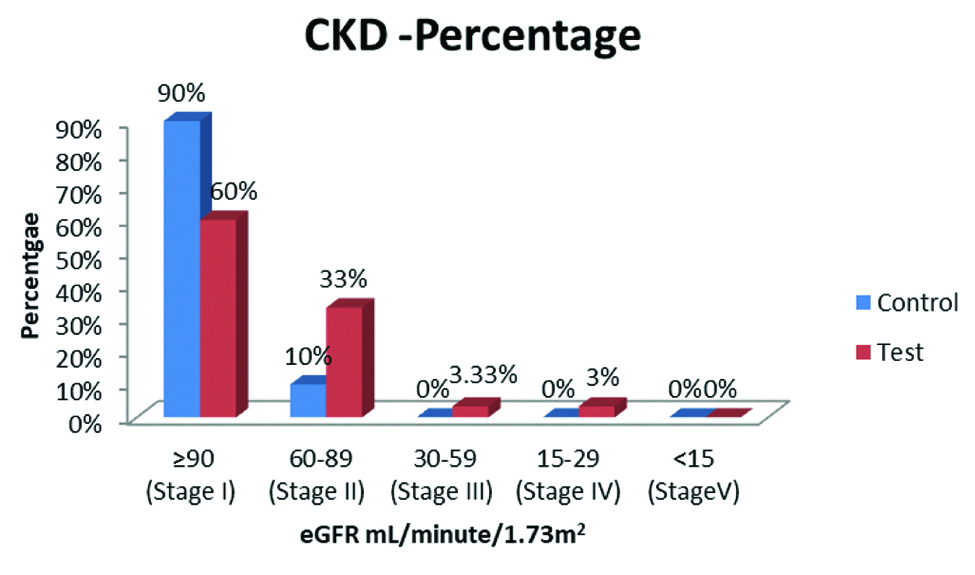
eGFR: eGFR was estimated based on serum creatinine level. In control group 90% of subjects were having eGFR >90 mL/minute/1.73 m2 and they were in Stage I (normal kidney function) whereas it was 60% in test group. About 40% of participants in test group had <90 mL/minute/1.73 m2 eGFR. Stage II to V indicates decrease in the kidney function. In present study there were no Stage V subjects. The stage of CKD and eGFR is shown in [Table/Fig-8].
The eGFR and stage of chronic kidney disease in percentage.
CKD: Chronic kidney disease; eGFR: Estimated glomerular filtration rate
From present study, it was observed that significant increase in serum creatinine was seen in PPI users. Significant difference was also seen with duration of PPI use and BUN levels. Decrease in kidney function based on eGFR values was observed in 40% of the test group. The change in renal parameters was not affected by regularity and type of use of PPI.
Discussion
The present study was designed to assess the renal function in patients on PPIs. PPIs are the widely used drugs for gastro oesophageal reflux disease and peptic ulcer. As these drugs act in the final step of gastric acid secretion by inhibiting the proton pump (H+K+ ATPase) which is responsible for the secretion of hydrogen ions into the gastric lumen where H+ ions combine with chloride to produce hydrochloric acid. Thus, PPI administration results in near complete suppression of acid secretion. This action of PPI provides significant relief from pain which mostly contributes to the repeated and long-term use of PPIs. The main advantage of these preparations is they do not lose their acid suppressing property even on long-term use [16].
The prevalence of peptic ulcer is found to be decreasing due to the use of PPIs as well as H2 blockers like Ranitidine and Famotidine [17].
Apart from the known adverse effects of PPI such as hyper-gastrinaemia and achlorhydria, hypomagnesemia and osteoporosis are reported recently. Studies on PPI use and renal status are limited especially in India.
The occurrence of peptic ulcer is reported to be higher in males than females and present study observations were in concurrence with this finding [18].
Peptic ulcer disease affects all age groups but rare in paediatric age group. In present study 26.6% of participants were above 18 and below 20 years of age, mostly students. In a cross-sectional study conducted on self-medication practice among under graduate medical students showed that antiulcer drugs (8.9%) were commonly used by students next to analgesic and antipyretics. The present study results also showed that majority of students were taking antiulcer drugs. It has been reported that gastritis is a common medical disorder among young adults due to their life style, food habits and stress [19].
In test group 23.3% were aged between 51 and 60 years. Peptic ulcer disease incidence increases with age and this could be the reason for the high percentage of participants in this age group found in present study. Helicobacter pylori infection is one of the risk factors for development of peptic ulcer. The prevalence of H. pylori infection has been found to peak at the age of 50 years [20].
After the age of 50, people may become susceptible for lifestyle disorders such as diabetes, hypertension and arthritis and they may be on multiple medications. It has become a common practice for the physicians to prescribe PPIs in order to prevent drug induced gastritis.
In present study we enrolled test group subjects who were taking PPIs only. Majority of the subjects were using PPIs regularly according to the prescribed duration. If symptoms recur after eight weeks, the patients require maintenance treatment with PPI which can be intermittent or continuous. Long-term therapy with PPI will itself predispose patients to be on continuous therapy because of rebound acid secretion [21]. According to Danish nation wise drug utilisation it has been reported that there was four-fold increase in number of users of PPIs (point prevalence) during the study period and it was more in elderly individuals [22].
NSAIDs are well known drugs to cause kidney damage. In present study, we excluded subjects who were on NSAIDSs. Most of the subjects in test group who were using PPI for more than six months were taking irregularly whenever they get symptoms of peptic ulcer disease. The reason for continuing PPI use in patients could be rebound-acid hypersecretion following withdrawal of PPI therapy.
Significant difference was observed in serum creatinine level between control and test group. About 13% of subjects had elevated serum creatinine level above normal range (>1.3 mg/dL). Serum creatinine is a byproduct of muscle metabolism that is excreted in unchanged from by the kidneys. An increase in serum creatinine can occur when kidney is not able to excrete properly. An increase in serum creatinine can also occur due to increased ingestion of cooked meat or excessive intake of protein and creatine supplements and intense exercise. However, this data was not taken for assessment in present study.
PPIs are considered as one of the common causes for drug induced interstitial nephritis. Patients prescribed PPIs have higher CKD risk than the normal population [9]. A nested case-control study showed a two-fold increased risk of AKI associated with PPI use in the previous 90 days [23].
The renal damage caused by PPI might be due to impairment of lysosomal acidification and proteostasis which in turn increases oxidative stress and dysfunction. PPIs also increase gene expression of heme-oxygenase-I which is a stress response protein [24].
The target for PPIs, H+K+ ATPase is present in gastric parietal epithelial cells and to a less extent in renal medulla. A pharmacoepidemiologic analysis on association of PPIs and the risk of dementia showed that avoidance of PPI may prevent the development of dementia. The proposed mechanism of brain damage could be due to non-selective blockade of sodium-potassium pumps in the brain causing osmotic imbalances or swelling in the cells [25].
The same mechanism can be proposed for kidney damage caused by PPIs. Kidneys have many such functioning pumps including sodium, potassium and chloride ion channels involved in ion balance, osmolality of body fluids and acid base balance. Inhibition of these ions channel will affect the renal function and homeostasis.
It has been reported that renal H+K+ ATPase pumps are also sensitive to omeprazole. Inhibition of this pump will affect not only the acid secretion but also potassium level [26]. Hypokalaemia has been reported to be a significant risk factor for progression of renal disease in CKD population [27].
It is well known that PPI use on long term cause hypomagnesemia. Tin A et al., observed in atherosclerosis risk in communities study that incident CKD was two-fold higher in individuals with low serum magnesium levels [28].
The accepted mechanism is that PPI is acting as a hapten and is taken up by dendritic cells of the kidneys. Subsequently Th2 subsets of T helper cell repertoire are activated leading on to eosinophilic infiltration and renal fibrosis causing kidney damage [29].
Even though the average serum creatinine level was within normal range, based on eGFR 40% of test subjects have decreased kidney function. Even though the sample size was less, only 60 % have normal eGFR. The term ‘Incident CKD’ is defined as the first sustained drop in eGFR below 60 mL/minute/1.73 m2 for at least 90 days [24]. In present study 6% of participants with eGFR below 60 mL/minute/1.73 m2 but these subjects were not followed for three months. The other common causes for kidney damage such as hypertension and diabetes were excluded and data obtained giva us the change in renal parameters was due to PPI use. To give definitive evidence, large sample size and base line renal parameters and estimation of electrolytes were required.
Significant correlation was seen with duration of use of PPI and BUN levels. This indicates that long-term use of PPI may affect the BUN levels. BUN values were within normal limits in test group. These values will be altered by extra renal factors such as high protein intake and catabolic states.
From present study, the results are giving a warning sign that these drugs should be used cautiously to reduce the incidence of drug induced kidney damage. Further studies with larger sample size are required, to establish their definitive role in causing renal damage.
Limitation
Small sample size: As the study was conducted under ICMR STS which was two month study, minimal sample size of 30 in each group was taken.
Base line renal parameters and electrolytes were not measured.
Conclusion
From present study it can be concluded that PPIs, irrespective of type whether used regularly or irregularly cause significant elevation of serum creatinine. It also caused decrease in eGFR in 40% of PPI users. It may be a warning sign that these drugs should be used cautiously to reduce the incidence of drug induced kidney damage. The change in renal parameters could be due to inhibition of H+K+ATPase in kidney or due to activation T helper cell causing immune damage. However, further studies with larger sample size are required to establish their role in causing renal damage.
*p-value significant
*p-value significant