High-risk pregnancy still remains a clinical challenge for many clinicians across the globe including India. Of the many representation of High-Risk Pregnancy condition including Gestational Diabetes Mellitus (GDM), Preeclampsia, BOH or PTB have been associated with high rates of foetal morbidity or mortality.
Preterm birth, defined as delivery at less than 37 weeks of gestation, complicates approximately 26% of pregnancies in India [1] and suggested to be suppressed or prevented with the complimentary use of progesterone supplementation in various forms or formulations. Though there have been several literatures on the therapeutic or interventional role of progesterone in high risk cases of threatened or preterm labour, the theoretical reasons why a foetus may survive but with disability has often been debated by the scientific fraternity. In such cases it is possible that an intrauterine mechanism that triggers preterm labour may also cause neurological injury to the foetus and that progesterone intervention may prevent labour but not foetal injury, further strengthening the concept note on prophylaxis strategy in such cases [1].
Progesterone is a hormone naturally produced by the corpus luteum and the placenta that offers hormonal and non-hormonal actions involving uterine quiescence and immunomodulation that are essential for maintaining pregnancy. Clinicians and researchers [1-3] have utlilised this hormone in its natural form to prevent PTB or habitual abortion, however, challenges still remain on the optimal timing, mode and dose of natural micronized progesterone particularly with sustained release formulations.
The use of injectable formulations including 17 α-hydroxyproge-steronecaproate (17P) as recommended by SMFM [4] clinical guidelines that is administered intramuscularly on a weekly basis is often compounded by site reactions involving irritation and discomfort not withstanding the compliance issues since the therapy is long-drawn requiring supplementation for treatment duration of upto 20 weeks.
Once daily sustained release formulations of natural progesterone offer oral dosage convenience for long-term compliance that is so vital in ensuring positive healthcare or infant and childhood outcomes while avoiding complications related to Low Birth Weight (LBW) and morbidity associated with NICU hospitalisation.
Materials and Methods
A Retrospective, case-cohort analyses of consecutive oral NMPSR prescriptions for BOH including PTB history, Threatened miscarriage or Habitual abortion utilising the DUS sheet was carried out at 40 outpatient centres across India in October’16 for these cases recorded between Jan’ to July ’16.
Data collection methods: The data was collected on patient demographics, obstetric history, medical and co-morbid diagnoses and relevant laboratory parameters including beta-hCG for above the diagnosis including Anti-Phospholipid Antibodies (APLA) investigations as applicable. Clinical notes on the risk assessment for delivery or pregnancy outcome at every follow-up visit upto 28 weeks or onwards by Trans Vaginal Ultrasound (TVUS) were sought and documented.
Data analysis: Statistical analyses were carried out by Quick Calcs Graph Pad Prism version 7 software with p-value <0.05 considered as significant for the continuing pregnancy rates @ 28 weeks or 34 weeks. Descriptive statistics was utilised to describe the numerical, categorical (nominal and continuous) data.
Results
Characteristics of patient cohort collected and collated during the study period of October 2016 included 185 patients who received oral NMP SR for High risk pregnancies as luteal phase support. Baseline demographics included mean age of 28.4 years, body weight 60.5 kg with mean abortion rate of 1% [Table/Fig-1]. However, for BOH cases with first or second trimester loss the, mean abortion rate was noted as 1.5 and 2 [Table/Fig-2,3] respectively.
Baseline demographics of High risk pregnancy cases.
| Patients (n) | 185 |
| Mean Age (yrs) | 28.4 |
| Mean Weight (kg) | 60.5 |
| Obstetric History | |
| G | 2.4 |
| P | 0.6 |
| A | 1 |
| L | 0.3 |
G: Gravida; P: Para; A: Abortion; L: Live
Baseline demographics for BOH women with first trimester loss.
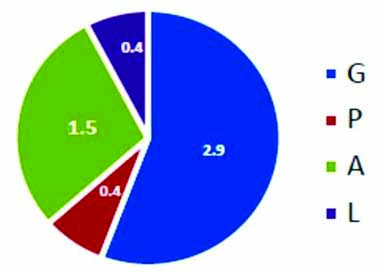
Baseine demographics for BOH women with second trimester loss.
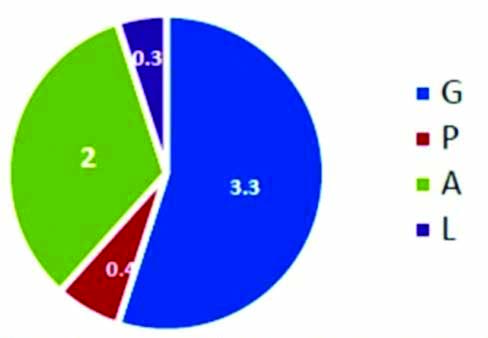
Natural progesterone as OMP SR was supplemented for PTB prophylaxis in BOH cases with first (n=36, 19.5 %) or second (n=37, 20%) trimester loss cases, Cervical factor (n=22, 11.9%), Still birth (n=15,8.1%), Threatened ± Spotting (n=19, 10.3), Placenta previa (n=5, 2.7%), PTB primary prophylaxis (n=22, 11.9%) & PTB secondary prophylaxis (n=12, 5.9%); Elderly primi ((2, 1.1%); Polyhydramnios (3, 1.6%); Uterine fibroid (3, 1.6%); Twin (7, 3.8%); Septate uterus (2, 1.1%).
Of the BOH cases, 50 women had preceding history of ≥2 pregnancy losses. Baseline demographics included mean age, 28.9 years, body weight 61.2 kg with mean abortion rate 2.4 [Table/Fig-4].
Baseline demographics for high risk population with BOH.
| Obs. h/o | Unexplained RPL (n=28) | Spontaneous loss (n=22) |
|---|
| G | 3.4 | 4.3 |
| P | 0 | 1.1 |
| A | 2.4 | 2.4 |
| L | 0 | 1 |
OMP SR was supplemented for BOH cases with Unexplained RPL (n=28, 56 %) or High risk cases of spontaneous pregnancy loss (n=22, 44%) with mean dosage of 271.4 and 286.4 mg for 18+5, and 19+1 week respectively.
Cases with ≥2 pregnancy losses on or before 24 weeks of gestation were labelled and assessed as unexplained RPL after ruling out Antiphospholipid Antibodies (APLA) syndrome as diagnoses. In all of these cases following supplementation with OMP SR, the pregnancies were followed up till 34th week with no reports of any adverse health outcome.
Similarly, 19 cases of spotting while receiving treatment for infertility were supplemented with oral NMP SR to prevent PTB as primary prophylactic strategy while in 12 other cases there was background history of preterm delivery where the same was given as secondary prophylaxis measure.
Clinical response: Oral NMP SR 300 mg was preferred in High-risk women with recurrent or singleton pregnancy loss; Cervical factor (incompetence or short cervix); Spot (Threatened miscarriage) as highlighted in [Table/Fig-4,5]. Oral NMP SR was preferred at varied dosages of 200 to 300 mg/day in women with PTB risk involving premature contractions or history.
Baseline clinical state and prescribed oral NMP SR dosage and duration.
| Clinical State | Oral NMP SR, Dose (Mean, mg) | Oral NMP SR, Duration (Mean, weeks) |
|---|
| Unexplained RPL | 271.4 | 18 + 5 |
| Cervical factor | 262.5 | 19.0 |
| Threatened miscarriage* | 311.1 | 10 +1 |
*Spotting or prior h/o
In all cases (100%), Pregnancy was continued till 34th week with no adverse outcome with two cases reported spotting (2, 1.1%) that were symptomatically managed.
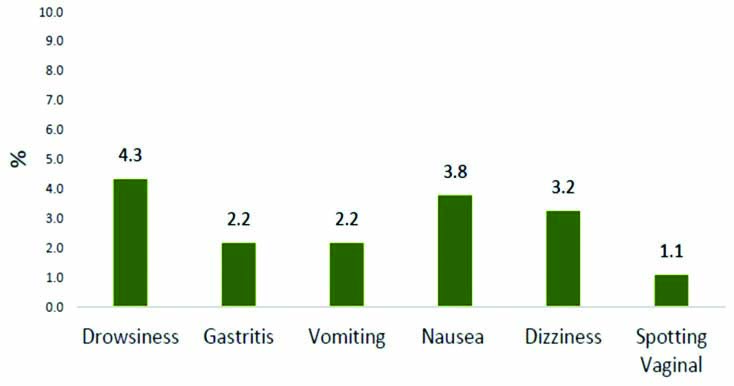
Safety profile: Treatment emergent adverse events included Gastritis (n=4, 2.2%), vomiting (n=4, 2.2%), drowsiness (n=8, 4.3%), dizziness (n=6, 3.2%), and treated symptomatically with none requiring further hospitalisation or referral. Spotting (n=2, 1.1%) [Table/Fig-6] was observed in two cases receiving 200 mg (Subchorionic haemorrhage) or 400 mg (Uterine fibroid with SCH) once a day respectively.
Adverse events observed with long term administration of OMPSR.
Discussion
The risk factors for PTB or preterm labour are many and difficult to predict as who would develop preterm labour. The strongest risk factor for PTB remains previous PTB, with others including being pregnant with twins, triplets, or more, history of cervical surgery (e.g., conization or cone biopsy) for abnormal Pap smears, if the amount of the cervix removed is large, uterine abnormalities, uterine bleeding especially in the second or third trimester, low pre-pregnancy weight and low weight gain during pregnancy, polyhydramnios, moderate to severe anaemia early in the pregnancy, and/or short interval (less than 12 to 18 months) between pregnancies. The current use of tocolytic agents or antibiotics has not been found to be effective in preventing PTB unlike progesterone. Natural progesterone with its immunomodulatory, anti-prostaglandin, & uterine relaxant properties plays an important role in secretory transformation of oestrogen primed endometrial lining to ensure adequate or proper oocyte implantation in high-risk cases of RPL playing a protective role in sustaining pregnancy till term [1,9].
In the current observational study, all of the high-risk pregnancy cases with a history of a previous PTB at less than 37 weeks (due to spontaneous unexplained pregnancy loss/labour.
Threatened miscarriage, Cervical factor including incompetence & short cervix, premature rupture of membranes) were supplemented with oral NMP SR to prevent recurrent PTB and related complications of increased neonatal morbidity or mortality. In most of the above cases, oral NMP SR was initiated between 16 and 26 weeks of pregnancy especially for High-risk women with second trimester loss or labour and continued until 34 weeks.
Several clinical studies [1-3] have evaluated the protective role of Immediate Release (IR) formulations of micronized natural progesterone at differing daily dosages of 100, 200 or 400 mg given orally to prevent recurrent PTB. The current study was the first one to assess the clinical response of SR formulation involving Matrix release technology in broad set of High-risk pregnancies involving BOH or Threatened miscarriage, Cervical factor, Still birth, PTB primary or secondary prophylaxis and Twinning. The most common preferred dosage in most cases remained as 300mg single dose of Oral NMP SR formulation. The results in the Unexplained RPL subgroup again confirms the clinical utility and safety of natural progresterone (SR) supplementation in this high-risk group as highlighted by Stephenson MD et al., Ismail AM et al., [10,11].
The formulation was well-tolerated with no clinically significant adverse events that required treatment withdrawal or further hospitalisation. Most of the adverse events were centrally mediated including Drowsiness (4.3%) or Dizziness (3.2%) that was well expected as reported by Purandare AC et al., as 0.6% when administered for BOH cases [12]. This is quite significant compared to the immediate release formulations of Natural progesterone that are usually taken orally often leading to higher incidence of sedation as reported by Frishman GN et al., when administered for Luteal Phase Defect (LPD) [13].
Limitation
The results of this clinical study were limited by the retrospective nature of the study design with natural progesterone supplementation for High-risk women including unexplained RPL documented to be timed around two to four weeks following biochemical or clinical confirmation of pregnancy. Further the findings of this observational, case cohort analyses have been exploratory for Oral NMP SR 300 mg formulation when utilised in High-risk pregnancies including Unexplained RPL that may be further confirmed by randomised or active controlled clinical trials involving peri-conceptional or conceptional use of supplementation.
Conclusion
High-risk pregnancy represents a clinical enigma with several management strategies suggested as prophylactic or therapeutic approach. Oral NMP SR can be suggested for ‘Primary’ or ‘Secondary’ prophylaxis strategy for High-risk pregnancies. Natural progesterone represents physiological yet safer option for long-term supplementation in these cases while avoiding complications of preterm delivery or infant mortality.
G: Gravida; P: Para; A: Abortion; L: Live
*Spotting or prior h/o