Alcoholic hepatitis is a clinical syndrome due to acute inflammation of liver parenchyma in patients with alcohol abuse and has high mortality of 30-60% [1]. Currently, CS, PTX and N-Acetyl Cysteine (NAC) are the therapeutic options available that may have mortality benefit [2-4]. Multiple prognostic scoring systems including MDF, MELD, CTP, Na-MELD, UKELD, ABIC and GAHS have been validated to assess the severity of AH and predict mortality risk in patients with SAH [5-9]. Lille score, early change in bilirubin level, increase in creatinine along with MDF and MELD are used for assessment of response to therapy after seven days [10-12].
Materials and Methods
This was a prospective comparative study conducted from December 2013 to January 2017 at Liver Care Unit, Osmania General Hospital, Hyderabad. This study was approved by in house Ethics committee and written informed consent was obtained from all the patients.
Inclusion criteria: Age 18 years or older, clinical AH with serum bilirubin >5 mg/dL, history of heavy alcohol abuse (>60 gm/day for males and >40 gm/day for females) for more than five years, AST/ALT ratio >2 with an AST level >45 but <500 U/L or ALT <300 U/L and AST/ALT ratio >2, MDF ≥32.
Exclusion criteria: Co-existent Chronic Liver Disease (CLD) (Non alcoholic steatohepatitis, iron load, biliary or autoimmune), recent hepatotoxic drug exposure, chronic viral hepatitis (Hepatitis B/C), biliary obstruction, malignancy, duration of current jaundice >2 months, patients dependent upon inotropic support (except terlipressin).
Data were collected prospectively from consecutive patients with diagnosis of SAH who satisfied inclusion/exclusion criteria. Patients were assessed at admission for severity of liver disease and presence of complications. MELD, CTP, MDF, ABIC, GAHS, UKELD, Na-MELD scores were calculated at the time of admission. MELD, MDF, CTP were re-calculated using laboratory data from day seven. SAH was defined as total bilirubin >5 mg/dL and MDF >32 in patients with history of active alcohol intake after ruling out other causes [15]. Acute renal failure was defined as abrupt reduction (48 hours) of renal function with an increase of 0.3 mg/dL in creatinine compared with baseline value [25]. All patients with normal renal function and without any complications or infection were started on CS (prednisolone 40 mg daily for four weeks followed by slow taper) and the rest were given PTX. CS were stopped and PTX given to those patients who developed infection, renal failure or any side-effects. Ascites, HE, HRS, Spontaneous Bacterial Peritonitis (SBP) and infections were treated as per standard guidelines. All patients received nutritional support with a diet containing 1.5 gm/kg protein and 35-40 kcal/kg energy and enteral feeding was instituted wherever required without delay.
Occurrence of death due to any cause within 30 days from hospital admission was the study endpoint.
Statistical Analysis
Survival analysis was carried out using the actuarial and Kaplan-Meier methods. Association with 30 day mortality for the individual variables and scores were calculated using Univariate Cox logistic regression analysis and significant parameters obtained were included in a multivariate Cox regression model. The Receiver Operating Characteristic (ROC) curves were graphed and best cut-off points for predicting 30 day mortality for each score were derived. Sensitivity, specificity, PPV and NPV of the models were calculated using originally published cut-offs. Comparison between AUROCs was performed by the method of Hanley JA and McNeil BJ [26].
Results
A total of 90 patients were admitted with clinical diagnosis of SAH. A total of 55 patients were included in the study after application of inclusion/exclusion criteria. The average alcohol consumption per day was 138.45±34.5 gm/day (80-220 gm/day) for at least five years in most patients.
Clinical characteristics and complications noted at the time of admission are shown in [Table/Fig-1]. Established cirrhosis based on ultrasound was documented in 19 patients (34.5%) and portal hypertension was present in 37 patients (67%). SBP was diagnosed in five patients (9%). Ascites was noted in 76.4%, HE in 40%, HRS in 18.2% and infections in 40%. Overall, 26 patients were started on CS and in seven patients CS were replaced with PTX therapy, while PTX was the main therapy in 29 patients. At the end of first week, six patients expired and four were discharged. Out of remaining 45 patients, 16 more died within 30 days. Mortality rate at 30 days was 40% (22 out of 55). Main cause of death was liver failure in 10 patients, sepsis/multi organ failure in eight and renal failure/HRS in four patients.
Characteristics of cases included (n=55).
| Variables | Mean±SD (range) |
|---|
| Demographic factors |
| Age | 46.9±7.7 (31-60) years |
| Male | 54 (98.2) |
| Duration of hospital stay | 15.76±6.5 (5-40) days |
| Alcohol (g/day) | 138.45±34.5 (80-220) |
| Clinical manifestations | n (%) |
| Jaundice | 55 (100%) |
| Fever | 19 (34.5%) |
| Oedema | 43 (78.2%) |
| Anorexia | 41 (74.5%) |
| Ascites | 42 (76.4%) |
| Asterixis | 22 (40%) |
| GI bleed | 18 (32.7%) |
| Hepatomegaly | 35 (63.6%) |
| Splenomegaly | 29 (52.7%) |
| Cirrhosis | 19 (34.5%) |
| Encephalopathy (HE) | 22 (40%) |
| HRS | 10 (18.2%) |
| Specific treatment | |
| Pentoxifylline | 29 (52.7%) |
| Corticosteroids | 19 (34.54%) |
| Corticosteroids->PTX | 7 (12.7%) |
GI: Gastrointestinal
All of the prognostic scores along with bilirubin, total protein/albumin, PT/INR, alkaline phosphatase, blood urea and creatinine were significant factors associated with 30 day mortality on univariate analysis as shown in [Table/Fig-2]. Among clinical parameters, only HRS (p<0.05) was significant whereas HE, sepsis, infections, leucocytosis, platelet count and Gastrointertinal (GI) bleed did not influence survival.
Univariate analysis of factors associated with mortality at 30 days.
| Variable | Hazard ratio | 95.0% CI | p-value |
|---|
| Lower | Upper |
|---|
| Age | 1.042 | 0.98 | 1.100 | 0.151 |
| Sex | 0.048 | 0 | 13248 | 0.635 |
| Alcohol (>140 gm/day) | 1.163 | 0.049 | 27.638 | 0.926 |
| Duration (>10 years) | 3.468 | 0.016 | 751.240 | 0.650 |
| Ascites | 0.763 | 0.51 | 1.13 | 0.176 |
| Asterixis | 0.056 | 0.016 | 0.191 | 0.500 |
| GI bleed | 0.933 | 0.302 | 2.88 | 0.904 |
| Hepatomegaly | 1.061 | 0.445 | 2.531 | 0.893 |
| Splenomegaly | 0.598 | 0.168 | 2.13 | 0.428 |
| HE | 1.130 | 0.794 | 1.619 | 0.490 |
| HRS | 0.135 | 0.057 | 0.322 | <0.001 |
| SBP | 0.580 | 0.194 | 1.73 | 0.329 |
| MOF | 0.502 | 0.118 | 2.14 | 0.352 |
| Infection | 0.006 | 0.289 | 0.12 | 0.695 |
| Sepsis | 0.253 | 0.068 | 0.932 | 0.390 |
| Hb | 0.047 | 0.000 | 86.839 | 0.426 |
| TLC | 1.003 | 0.993 | 1.013 | 0.528 |
| PMN | 0.197 | 0.000 | 137.990 | 0.627 |
| Platelet | 1.000 | 0.998 | 1.002 | 0.935 |
| Bilirubin | 1.550 | 1.3 | 1.84 | <0.001 |
| ALT | 1.244 | 0.230 | 6.734 | 0.800 |
| AST | 1.033 | 1.008 | 1.058 | 0.008 |
| ALP | 0.868 | 0.78 | 0.955 | 0.004 |
| TP | 0.019 | 0.003 | 0.118 | <0.001 |
| Albumin | 0.074 | 0.22 | 0.249 | <0.001 |
| PT | 1.26 | 1.17 | 1.35 | 0.002 |
| INR | 17.2 | 7.17 | 41.6 | <0.001 |
| Urea | 1.139 | 1.092 | 1.18 | 0.005 |
| Creatinine | 5.33 | 2.83 | 10.09 | 0.004 |
| RBS | 1.003 | 0.987 | 1.018 | 0.752 |
| Na+ | 0.892 | 0.84 | 0.947 | 0.007 |
| K+ | 1.369 | 0.81 | 2.29 | 0.231 |
| MDF | 1.048 | 1.033 | 1.063 | 0.003 |
| CTP | 2.55 | 1.85 | 3.51 | 0.005 |
| MELD | 1.339 | 1.232 | 1.455 | 0.003 |
| NA_MELD | 1.42 | 1.27 | 1.59 | 0.003 |
| ABIC | 3.76 | 2.39 | 5.92 | <0.001 |
| GAHS | 4.6 | 2.85 | 7.44 | <0.001 |
| UKELD | 1.31 | 1.19 | 1.43 | 0.005 |
CI: Confidence interval; GI: Gastrointestinal; INR: International normalised ratio; MOF: Multi-organ failure
On multivariate analysis amongst prognostic scores which were found significant on univariate cox regression analysis, UKELD (p<0.01, Hazard Ratio (HR)-1607.39), CTP (p<0.01, HR-607.35) and MDF (p<0.01, HR-6.31) showed more significance compared to MELD, Na-MELD, ABIC or GAHS [Table/Fig-3].
Multivariate cox regression analysis of parameters with significant p-value for mortality at 30 days.
| Variable | p-value | Hazard ratio | 95.0% CI for Exp (B) |
|---|
| Lower | Upper |
|---|
| HRS | 0.34 | 11.15 | 0.08 | 1.63 |
| Bilirubin | 0.02 | 18.12 | 0.13 | 10.04 |
| AST | <0.001 | 1.14 | 1.04 | 1.25 |
| ALP | 0.52 | 0.78 | 0.65 | 0.93 |
| TP | 0.14 | 0.00 | 0.00 | 0.19 |
| Albumin | 0.78 | 44.60 | 0.00 | 2.53 |
| PT | 0.29 | 3.77 | 0.32 | 44.45 |
| INR | 0.01 | 0.00 | 0.00 | 0.00 |
| Urea | <0.001 | 79.34 | 3.87 | 16.28 |
| Creatinine | 0.01 | 0.00 | 0.00 | 0.00 |
| Na+ | 0.05 | 15.07 | 0.68 | 331.90 |
| MDF | 0.01 | 6.31 | 1.43 | 27.80 |
| CTP | 0.01 | 607.35 | 5.85 | 6.30 |
| MELD | 0.25 | 27.47 | 0.09 | 8.16 |
| NA_MELD | 0.92 | 0.55 | 0.00 | 4.03 |
| ABIC | 0.26 | 0.00 | 0.00 | 8.74 |
| GAHS | 0.81 | 7.60 | 0.00 | 15.64 |
| UKELD | 0.01 | 1607.39 | 8.92 | 28.96 |
CI: Confidence interval; INR: International normalised ratio
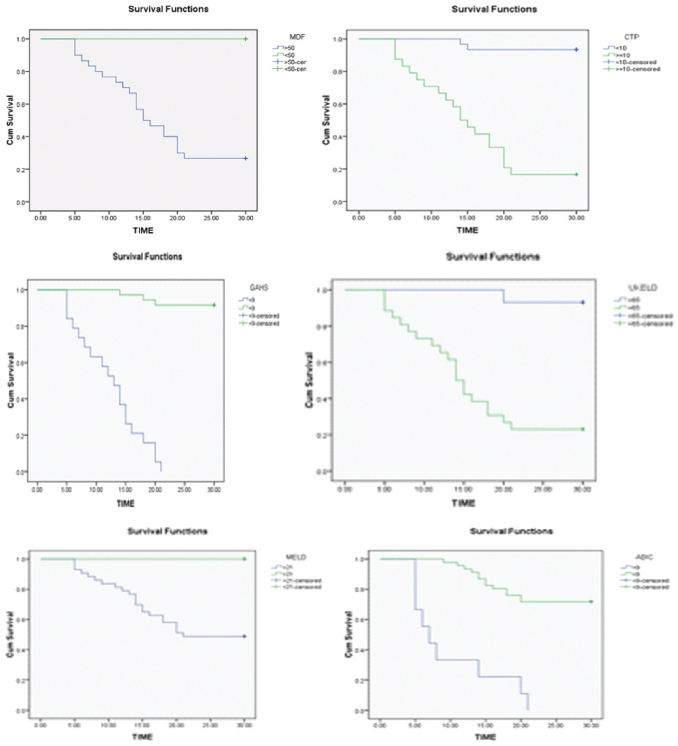
Mortality was significantly higher with MELD score >21 and MDF >50 with the Kaplan Meier (K-M) survival curves showing a probability of only 50% survival at 22 days in patients with MELD >21 and only 30% survival in those with MDF >50. CTP score of 10 or more also showed significant association with 30 day mortality. ABIC and GAHS of 9 or more were significant (p<0.05) with K-M analysis showing >90% mortality with both scores, however patients with ABIC score of <9 still had significant risk of 30% mortality at 20 days. Patients with UKELD score >65 showed a survival probability of only 20% at 22 days [Table/Fig-4a-f].
Kaplan Meier (K-M) survival analysis of MDF, CTP, GAHS, UKELD, MELD and ABIC scores for 30 day mortality.
The area under curve for admission MELD was highest at a cut-off value of 25.9 with an AUROC of 1 but not statistically different compared to both CTP and MDF. The AUROCs for the prediction of 30 day mortality ranged from 0.933 for UKELD and ABIC to 1.00 for MELD and 0.997 for MDF. No significant differences were found in pairwise comparisons between the AUROCs of the different models [Table/Fig-5].
Receiver-Operating Characteristic (ROC) curves of different scores.
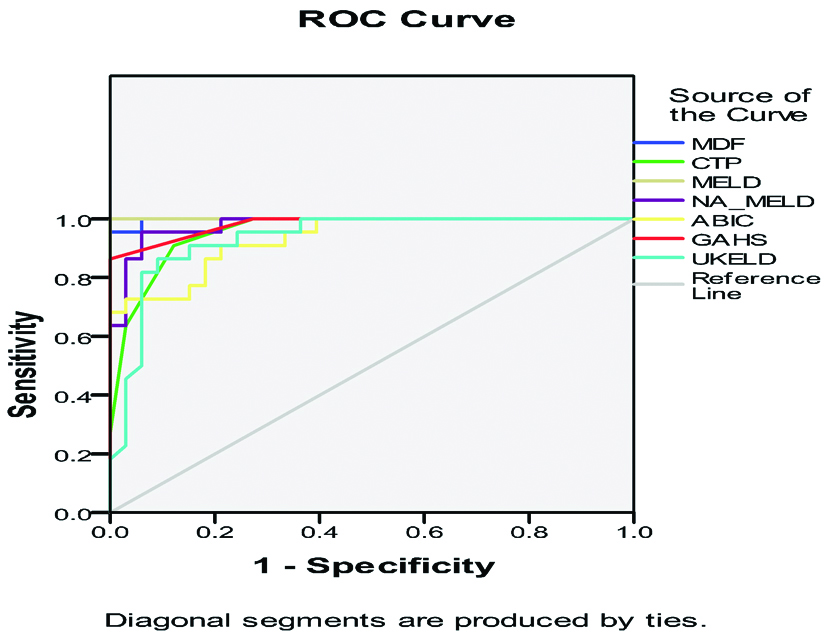
Sensitivity and specificity were calculated at the best cut-off value at the highest point of the curve. The cut-off values obtained are shown in the [Table/Fig-6]. Using the proposed cut-off points, the specificity and PPV for 30 day mortality were high (most exceeding 90%) with MDF, MELD and GAHS having 100% PPV and specificity while ABIC had least PPV of 76.92% at a cut-off value of 7.66. At these cut-off values, the sensitivity and NPV of the scores were significantly higher with most scores having >90% sensitivity.
Best cut-off value, sensitivity/specificity, PPV/NPV of all scores measured at time of admission.
| Test result variable | Area under curve | p-value | Best cut-off value | Sensitivity | Specificity | PPV | NPV |
|---|
| MDF | 0.997 | <0.001 | 67.50 | 95.50 | 100.00 | 100 | 97.6 |
| CTP | 0.956 | <0.001 | 9.50 | 90.90 | 87.90 | 83.3 | 93.5 |
| MELD | 1.000 | <0.001 | 25.90 | 95.50 | 100.00 | 100 | 97.06 |
| NA_MELD | 0.978 | <0.001 | 29.10 | 95.50 | 93.90 | 95.4 | 96.9 |
| ABIC | 0.933 | <0.001 | 7.66 | 90.90 | 78.80 | 76.92 | 93.1 |
| GAHS | 0.981 | <0.001 | 9.50 | 86.40 | 100.00 | 100 | 91.67 |
| UKELD | 0.933 | <0.001 | 65.60 | 86.40 | 90.90 | 86.36 | 90.9 |
Re-scoring of MELD, MDF and CTP at day seven generally yielded a trend towards increased AUROCs [Table/Fig-7]. MELD at a cut-off of 28 at 7 days showed 100% specificity with a PPV of 100 while MDF with best cut-off of 59.7 at 7 days had 100% sensitivity and a NPV of 100 as shown in [Table/Fig-8]. However, CTP did not show significant dynamic change at day seven.
Receiver-Operating Characteristic (ROC) curves of different scores measured at day seven.
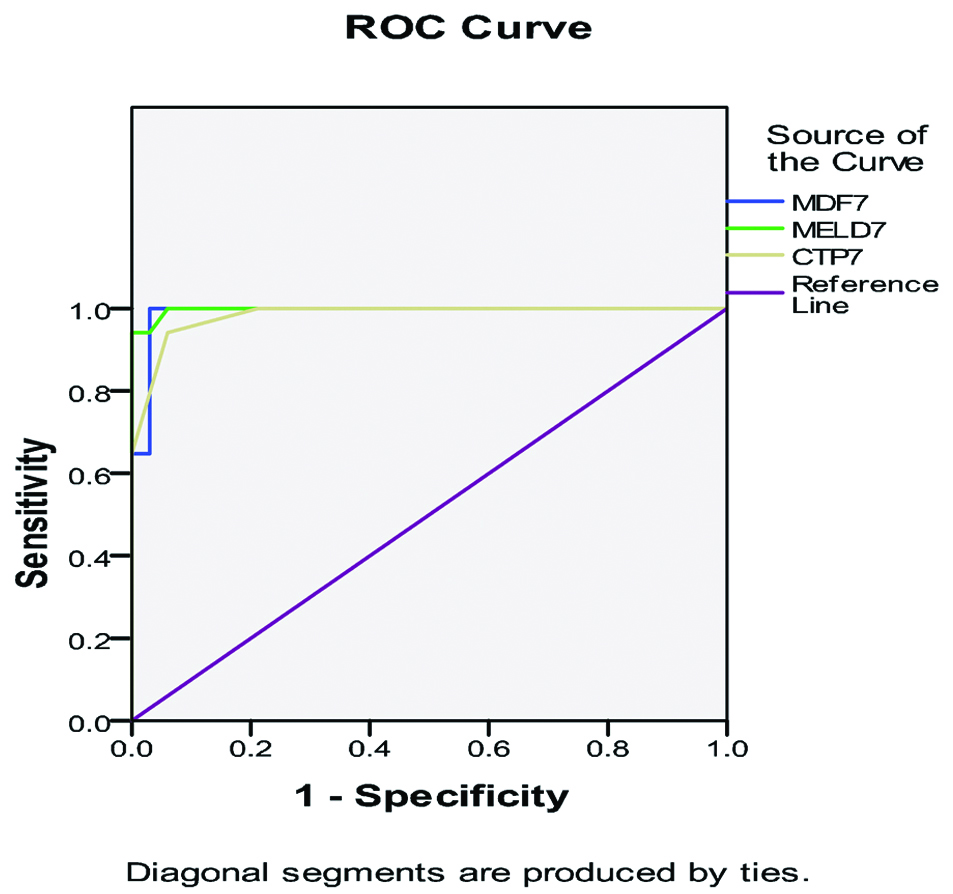
Best cut-off value, sensitivity/specificity, PPV/NPV of CTP, MDF and MELD measured at day seven.
| Variable | Area | p-value | Best cut off | Sensitivity | Specificity | PPV | NPV |
|---|
| MDF7 | 0.989 | <0.001 | 59.7 | 100 | 97 | 94.44 | 100 |
| MELD7 | 0.997 | <0.001 | 28 | 94.1 | 100 | 100 | 97.4 |
| CTP7 | 0.983 | <0.001 | 9.5 | 94.1 | 93.9 | 88.9 | 98.18 |
Discussion
Multiple validated prognostic scoring systems exist in SAH but very few studies have compared validity of prognostic scores in Indian patients. This prospective study was designed to assess and compare utility of various prognostic scores in predicting 30 day mortality in an Indian cohort of patients with SAH. In present cohort, the 30 day mortality was 40%, which was consistent with previous studies reporting short-term mortality ranging to 14.4-57% [5-9]. Among clinical variables, 30 day mortality was not significantly associated with HE, ascites, sepsis and age compared to HRS (Hazard Ratio (HR)-0.135 (p<0.05)). Bilirubin, INR, Na+, urea and creatinine all showed significance (p<0.05) on multivariate analysis reflecting the fact that CTP, MDF and UKELD also were found to be significant. In a study by Higuera-de la Tijera F et al., the main factors associated with mortality in patients with SAH were, underlying cirrhosis and development of HE [27]. This is corroborated by very high significance of CTP {(HR)-607.5 (p<0.01)} in this study, however, HE was not significant. Optimal cut-off for MDF within present cohort for identifying patients with high 30 day mortality corresponds to MDF score of >50 (p<0.05) at day one, which showed probability of only 30% survival at 22 days. MDF with best cut-off of 67.5 at day one had 100% specificity and a PPV of 100 while a cut-off of 59.7 at day seven had 100% sensitivity and a NPV of 100 for early mortality. A MELD score of 25.9 on admission with an AUROC of 1.00 (sensitivity/specificity- 95.5/100%) and day seven MELD of 28 with an AUROC of 0.997 (sensitivity/specificity-94.1/100%) has been identified as one of the best predictors of early mortality in present cohort; this is consistent with results from the study by Bargalló-García A et al., who identified the MELD score as the best scoring system [28]. In present study, UKELD score of 65 (HR-1607.39 (p<0.005)) had significant p-value (<0.05) for 30 day mortality with K-M Survival curve showing a survival probability of only 20% at 22 days. All patients who died during hospitalisation presented high risk ABIC, and all patients grouped as low risk survived. ABIC and GAHS scores of 9 were also associated with high mortality at 30 days (p<0.05). However, ABIC patients with score of <9 still had significant risk of 30% mortality at 30 days. An important distinction can be made in patients with GAHS <9 but MDF >32 in that they have good prognosis with therapy with most responding to treatment.
At higher cut-off values, all prognostic models showed excellent sensitivity, specificity, PPV and NPV with NPV in most cases exceeding 90%. MELD score of 25.9 at day one and 28 at day seven, MDF score of 67.5 at day one and GAHS of 9.5 had the best specificity and PPV of 100% where as ABIC and CTP had least PPV of 76.9 and 83.3 at cut-off values of 7.66 and 9.5 respectively. Similar to previous studies [7,17,23,24], this study showed excellent NPV for all scores while specificity and PPV also increased albeit with higher cut-off values, suggesting that at higher cut-off values, scores like MDF, MELD, UKELD can be used to stratify risk at an early stage while the excellent NPV and sensitivity of the scores should be able to exclude low-risk patients from possibly unwarranted therapy.
To summarise, all the current scores are effective in stratifying 30 day mortality risk in SAH with good sensitivity as well as specificity with an admission MDF >67.5, MELD >25.9, UKELD >65, GAHS >9.5 or more conferring very high risk of 30 day mortality and these patients require intensive care and early consideration for liver transplantation or use of newer therapies that may improve survival.
Limitation
The sample size of 55 in this study is relatively small and may not accurately predict differences among various scores compared. Lack of biopsy is an important limitation of present study and diagnosis of cirrhosis was based on ultrasonography findings. Patients with SAH requiring inotropes at admission were not included.
The current prognostic scores are limited by differing cut-offs for risk stratification, initiation of therapy and lack of dynamic assessment. Therefore, efforts for improved prognostic models should continue with inclusion of new biomarkers, combining static and dynamic models to further refine prognostic stratification and early identification of non-responders, particularly with the option of early liver transplantation. Also, studies from multicentre units with large sample size are required to verify these findings.
Conclusion
United kingdom end stage liver disease and CTP scores calculated at the time of admission showed most significance on all the validated scores in prediction of 30 day mortality. ABIC and GAHS scores above 9 confer a very high early mortality risk, however those with MDF>32 but GAHS<9 do respond well to therapy. At higher cut-off values, MDF, MELD, UKELD calculated at day one and MDF, MELD calculated at day seven can be used to accurately identify patients at high risk of death and these patients may be considered early for liver transplantation that may prolong survival as they have high mortality risk even with standard therapy as per current guidelines.
GI: Gastrointestinal
CI: Confidence interval; GI: Gastrointestinal; INR: International normalised ratio; MOF: Multi-organ failure
CI: Confidence interval; INR: International normalised ratio