Introduction
Pregnancy is a remarkable phenomenon, however 15-25% of pregnancies end up in sporadic abortions. Less than 5% and 1% of women experience two and three consecutive pregnancy losses respectively [1]. The American Society of Reproductive Medicine defines RPL as a distinct disorder characterised by two or more documented clinical pregnancy losses excluding biochemical losses (not necessarily consecutive) [2]. European Society for Human Reproduction and Embryology [3] refers it as two or more pregnancy losses and the Royal College of Obstetricians and Gynaecologists [4], refer it as three consecutive pregnancy losses, including non-visualised ones. The expected probability of having three consecutive spontaneous abortions is about 0.3-0.4% however, the epidemiological studies have shown higher incidence [5]. The prevalence of RPL differs among international societies, as definition varies, affecting 2-5% of couples [6]. Miscarriage or spontaneous abortion is a loss of a clinical pregnancy before 20 completed weeks or the loss of an embryo/foetus of <400 gm [7]. European Society of Human Reprodction and Embryology (ESHRE) mentions it as pregnancy loss before 24 weeks or before the age of viability, which is different in different countries [8].
Primary RPL indicates two or more pregnancy losses in a woman who has not had a pregnancy beyond the age of viability; secondary RPL means multiple pregnancy losses in a women, who has a pregnancy beyond the age of viability and tertiary RPL indicates many pregnancy losses before and after the normal pregnancies [9].
The definition, investigations, and management of RPL are one of the most debated topics. This study was aimed to provide an overview of aetiologies, work-up, and an evidence-based approach to manage RPL.
Aetiologies and Evaluation for RPL
1. Cytogenetic Abnormalities
Up to 60% of sporadic early miscarriages are attributed to chromosomal abnormalities (aneuploidies) [10]. Genetic abnormalities leading to pregnancy loss include chromosomal aberrations (numerical and structural), and gene mutations. Amongst them, the most common parental abnormality is balanced translocations, found in 3-5% of cases of RPL, compared to 0.7% in the general population [11]. Up to 60% are seen as reciprocal translocation, affecting non-homologous chromosomes and rest are Robertsonian translocations, involving acrocentric chromosomes. Paracentric and pericentric inversions are less commonly observed [11]. Other karyotypic abnormalities are ring chromosomes, deletions and duplications and mosaicism.
2. Structural Uterine Defects
Uterine anomalies are found in 19% of women with RPL [12] and are acquired or congenital. Congenital Uterine Anomalies (CUA) are found in 8.4-12.6% of women with RPL compared to 1-1.5% in general population [13]. The septate uterus is the most common CUA associated with spontaneous miscarriages [14]. Other anomalies like unicornuate, bicornuate and uterine didelphys are associated with late pregnancy losses and preterm birth [14].
Acquired uterine anomalies are leiomyoma, polyp and intrauterine synechiae (adhesions), clinical significance for RPL association is unclear [15]. Intrauterine synechiae occurs when the endometrial basal layer has been destroyed frequently following curettage, other causes are uterine surgery or infection, or a complicated birth [16]. Submucosal myomas and polyps are found in 4.5% of women and 2-3% of women with RPL respectively [13]. Cervical incompetence usually causes second trimester losses, and it can be acquired following surgical trauma or is associated with CUA [13].
3. Immunological Abnormality
Because a foetus is genetically not identical to the only mother, an immunological modification is necessary to prevent immune rejection. Multiple immunogenic causes have been proposed. The autoimmune condition of importance for RPL is Antiphospholipid Syndrome (APS), which is an acquired thrombophilia. It accounts for 5-20% of cases of RPL, though it varies widely (5-42%) [17]. The clinical criteria for the diagnosis of APS in RPL is an occurrence of three or more consecutive pregnancy losses before 10th week of gestation, in absence of parental chromosomal, anatomic and hormonal abnormalities [18]. The antiphospholipid antibodies are acquired and it damages trophoblast, leading to impaired trophoblast mediated functions like spiral artery formation, secretion of growth factors and human Chorionic Gonadotrophin (hCG), early apoptosis of trophoblasts and abnormal inflammatory response leading to impaired pregnancy support [19].
4. Endocrine and Metabolic Factors
The consensus is that maternal diabetes and thyroid abnormality have been associated with RPL. Uncontrolled diabetes increases the risk of miscarriage, whereas an adequate pre-conception control significantly reduces risk back to normal. A direct correlation is seen between the level of glycosylated haemoglobin and the early abortion [2]. Subclinical and clinical hypothyroidism is shown to be associated with RPL.
Polycystic Ovarian Syndrome (PCOS) is associated with an increased risk of miscarriage. Many mechanisms thought to be involved like insulin resistance and hyperinsulinemia, hyperandrogenemia, or increased plasminogen activator inhibitor-1 activity [20]. Luteal Phase Deficiency (LPD) has been proposed as a cause of early miscarriage and RPL, but its definition and true impact on pregnancy rates remain highly controversial [6].
5. Other Speculative Causes
a) Infectious agents: The prevalence of endometritis has been reported as high as 58% in patients with RPL. The presence of plasma cells in endometrial sample confirms the presence of endometritis. The type of sampling and the mode of investigation like the use of hysteroscopy and immunohistochemistry (for antibodies to CD138) etc., may affect the prevalence. Endometrial permissiveness to embryo can be disturbed by check point abnormality. Further well designed studies are needed to prove its clinical effect [6]. For an infective agent to be implicated in the aetiology of RPL, it should be persistent in the genital tract and must cause sufficient symptoms to disturb female which allows its detection. Toxoplasmosis, rubella, herpes, cytomegalovirus infection and Listeria do not fulfill criteria and hence, routine testing for Toxoplasmosis, Rubella, Cytomegalo virus, Herpes simplex (TORCH) screening is not recommended. Screening for bacterial vaginosis is indicated in women with previous history of second trimester abortion and preterm labor. Ureaplasma urealyticum, Mycoplasma hominis, Chlamydia, Listeria monocytogenes are frequently found in vaginal and cervical cultures with sporadic abortions. However, there are no convincing data that infections cause RPL [2].
b) Inherited thrombophilia: Inherited thrombophilia refers to the conditions that increase the risk of thromboembolism, secondary to genetic alteration of a functional protein in the coagulation cascade. Most common thrombophilia are factor V Leiden mutation, prothrombin gene mutation, activated protein C resistance and methyl tetrahydrofolate reductase mutation. Deficiency of protein S/C and antithrombin are less common in general population [21]. It is reported weakly associated with RPL [6].
c) Male factors: The sperm quality, smoking, alcohol, exercise, body weight and occupational hazards can have effect on sperm quality. Sperm DNA fragmentation is seen to be consistently related to RPL in few studies. It is found to be associated with natural and IVF conception. The moderate association has been established between sperm DNA damage and RPL. Standard semen parameters do not predict the pregnancy loss. The aneuploidy of sperm is studied for RPL [2]. It is more useful for unexplained RPL couples.
d) Psychological, life style, environmental and occupational issues: There are insufficient data to support psychological component in aetiology of RPL. Obesity is associated with an increased risk of RPL. Alcohol, caffeine, cocaine, cigarette smoking and stress has been associated with risk of miscarriage [2].
e) Allo-immune factors: HLA sharing at A, B, C and DR loci by couples may be associated with increased risk of abortion that results from an absence of maternal blocking antibodies. Other immunogenic factors such as HLA G polymorphism, antipaternal antibody levels, embryotoxic factors and decidual cytokine profiles have shown inconsistent results for RPL evaluation [2].
6. Unexplained RPL
Despite detailed investigations, 50-60% of cases with RPL remain unexplained [2]. This includes genuine RPL by chance and pathologic unexplained RPL which are not identified by an available investigative protocol.
Workup of RPL: It is now accepted to start a workup following two consecutive losses [12]. It is of importance particularly in anxious, elderly and a women with infertility. In cases of mid second trimester losses, evaluation should be initiated immediately after one pregnancy loss as aetiology is more amenable to early diagnosis [2]. The evaluation must be tailored according to a woman and her partner’s age, the personal and family medical history, the couple’s emotional state, technical platform, and the finances [2]. An essential workup of RPL includes genetic, anatomic, immunogenic and endocrine evaluation. Additional testing is advised only if indicated by history and physical examination [Table/Fig-1].
Workup of RPL in absence of product of conception analysis.
NK: Natural killer; HLA: Human leukocyte antigen; PCOS: Polycystic ovarian syndrome;
ANA: Antinuclear antibodies
*refer to [Table/Fig-2] for diagnostic modalities and strength of test for RPL.
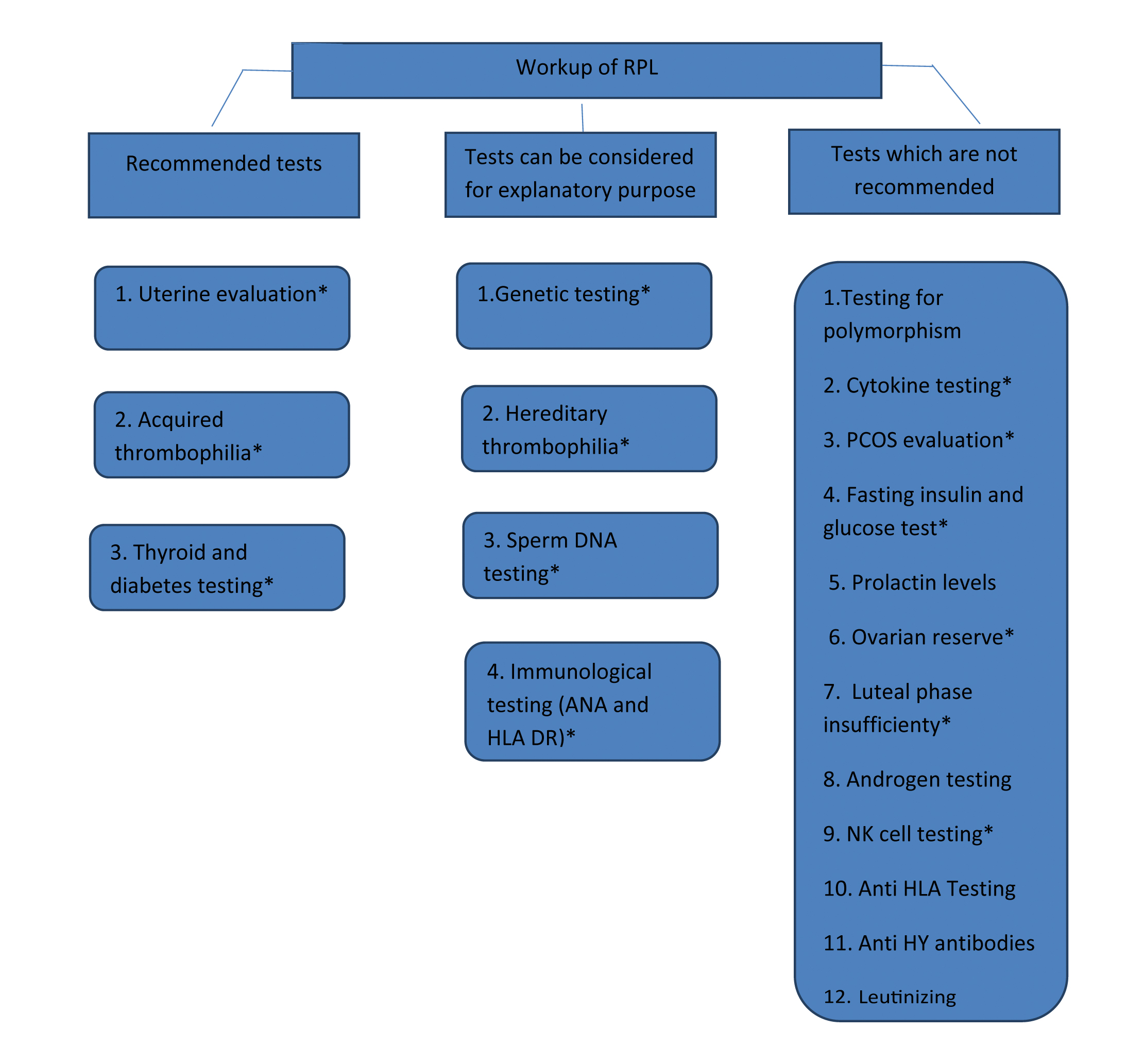
[Table/Fig-2] shows various tests and treatment recommendation. Recommended tests are glucose tolerance test (or haemoglobin A1c) and serum Thyroid Stimulating Hormone (TSH) and antibodies for Antiphospholipid Antibody Syndrome (APS). Most suitable screening test for Lupus anticoagulant is activated partial thromboplastin time and dilute Russel’s viper venom test. Highly sensitive and specific test like ELISA for IgG, IgM antibodies are most widely used for cardiolipin antibodies and β2 glycoprotein I antibodies. Screening tests for many other antiphospholipid antibodies like phosphatidyl serine, phosphatidyl ethanolamine, phosphatidylinositol are not yet standardised [2]. TSH value more than 2.5 mIU/L is considered above the normal upper limit, which is to be further evaluated by free T4 and antithyroid antibody levels [22]. It is recommended to keep upper levels of TSH <2.5 mIU/L [22]. Testing for thyroid autoantibodies is only recommended if abnormal TSH is found. Prolactin levels are measured only if it is associated with ovulatory dysfunction (oligomenorrhoea/amenorrhoea). Testing for inherited thrombophilia is not recommended unless there is a personal (thrombosis in the absence of trigger) or a strong family history of thrombosis [2,23].
Suspected causes and recommendation for recurrent pregnancy loss evaluation and treatment.
| Factors | Diagnostic modalities | SpecificIndications in RPL | Association | Contribution | Prognosis | Treatment | Recommendation for testing |
|---|
| Genetic analysis |
| a) Pregnancy tissue | Array CGHKaryotype | 2nd and 3rd PL | Yes (strong) | Yes | No | | Can be considered |
| b) Parental testing | Karyotype | | Yes | Yes | Yes | Counseling,PIGS, PIGD and PND | Can be considered |
| Thrombophilia |
| a) Hereditary | Factor V Leiden, MTHFR, Prothrombin gene mutation, Protein C, S and antithrombin | Only with a family history of thrombophilia, personal history of VTE without triggering factor | No/weak | Unclear | Yes | Not to use antithrombotic prophylaxis | Not recommended |
| b) Acquired | LA, ACL* | | yes (strong) | Yes | Yes | Heparin and low dose aspirin | Recommended |
| Alpha 2 beta globin | | Possible | Possible | No data | No data | Can be considered |
| ANA | | Yes | Probably not | Unclear | | Can be considered |
| Endocrine and metabolic |
| a) Hypothyroidism | TSH* | | Only sporadic | Only sporadic | Yes | levothyroxine | Recommended |
| b) Subclinical hypothyroidism | TSH | | Yes | Yes | Not clear | Unknown if efficient | Recommended |
| c) Hyperthyroidism | TSH | | No | No | Not clear | Yes | Recommended |
| TPO antibodies* | | Yes (strong) | YesWith TPO | Yes | Studies needed | Recommended |
| TG antibodies | | No | Yes | Yes | Studies needed | Not recommended |
| d) Diabetes | Hemoglobin A1c | | Yes | Yes | Yes | Yes | Recommended |
| e) PCOS* | Fasting sugar and insulin | Oligo/amenorrhoea | Yes (weak) | No data | No studies | Yes | Not recommended |
| f) Prolactin* | S. Prolactin | | Inconsistent | No data | Possible | Yes | Not recommended |
| g) Ovarian reserve* | S. Progesterone | | No evidence | No data | ------- | No studies | Not recommended |
| h) Luteal phase defect* | Luteal Endo-metrial biopsy | | Inconsistent | | No data | Possible | Not recommended |
| Uterine factors |
| a) Congenital uterine anomaly | 3D ultrasound*MRI, Hysteroscopy, SHG | | Yes | Some malformations | | targeted resection | Recommended |
| b) Acquired conditions | As above | | Unclear | Unclear | | Unclear | -------- |
| Male factors |
| a) DNA fragmentation* | ----- | | Moderate | probably | Unclear | NO | Can be considered |
| Other factors |
| A) Maternal HLA determination* | HLA-DQB1* | Ethnic selection | Strong | Yes | Negative impact | None available | Not recommended |
| B) HLA compatibility | HLA-A, B, C, DR | | Controversial | NA | No | None available | Not recommended |
| C) Cytokines | | | Yes | Unclear | Unknown | NA | Not recommended |
| D) NK cells | Peripheral blood and endometrium | | Weak | No | Unclear | No | Not recommended |
| E) Vitamin D | Blood levels | | Possible | Possible | Unknown | Yes | Not recommended |
| F) Obesity | | | Moderate | | | Life style | |
| G) alcohol | | | Weak/moderate | | | modification | |
CGH: Comparative genomic hybridisation; MTHFR: Methyl tetra hydro folate reductase; LA: Lupus anticoagulant; ACA: Anti cardiolipin antibody; ANA: Antinuclear antibody; TSH: Thyroid stimulating hormone; TPO: Thyroperoxidase antibodies; TG: Thyroglobulin; 3D: 3 Dimensions; MRI: Magnetic resonance imaging; SHG: Sonohysterogram; HLA: Human leucocyte antigen; * labeled tests are recommended only in selected cases
Imaging: A transvaginal two dimensional Ultrasound (US) for the assessment of the uterus is widely recommended for primary evaluation, as it is readily available and quick. The preferred technique is transvaginal 3D US. Other modalities to assess uterine cavity are sonohysterography (preferred on hysterosal pingography), which is non-invasive, and hysteroscopy along with laparoscopy, which is invasive testing. Pelvic magnetic resonance imaging is recommended only where the 3D US is not available; as second line option. It is highly specific and sensitive method available because of its superior ability to reliably visualise complex utero-vaginal anatomy [2]. Testing for chronic endometritis is not recommended in the absence of strong evidence.
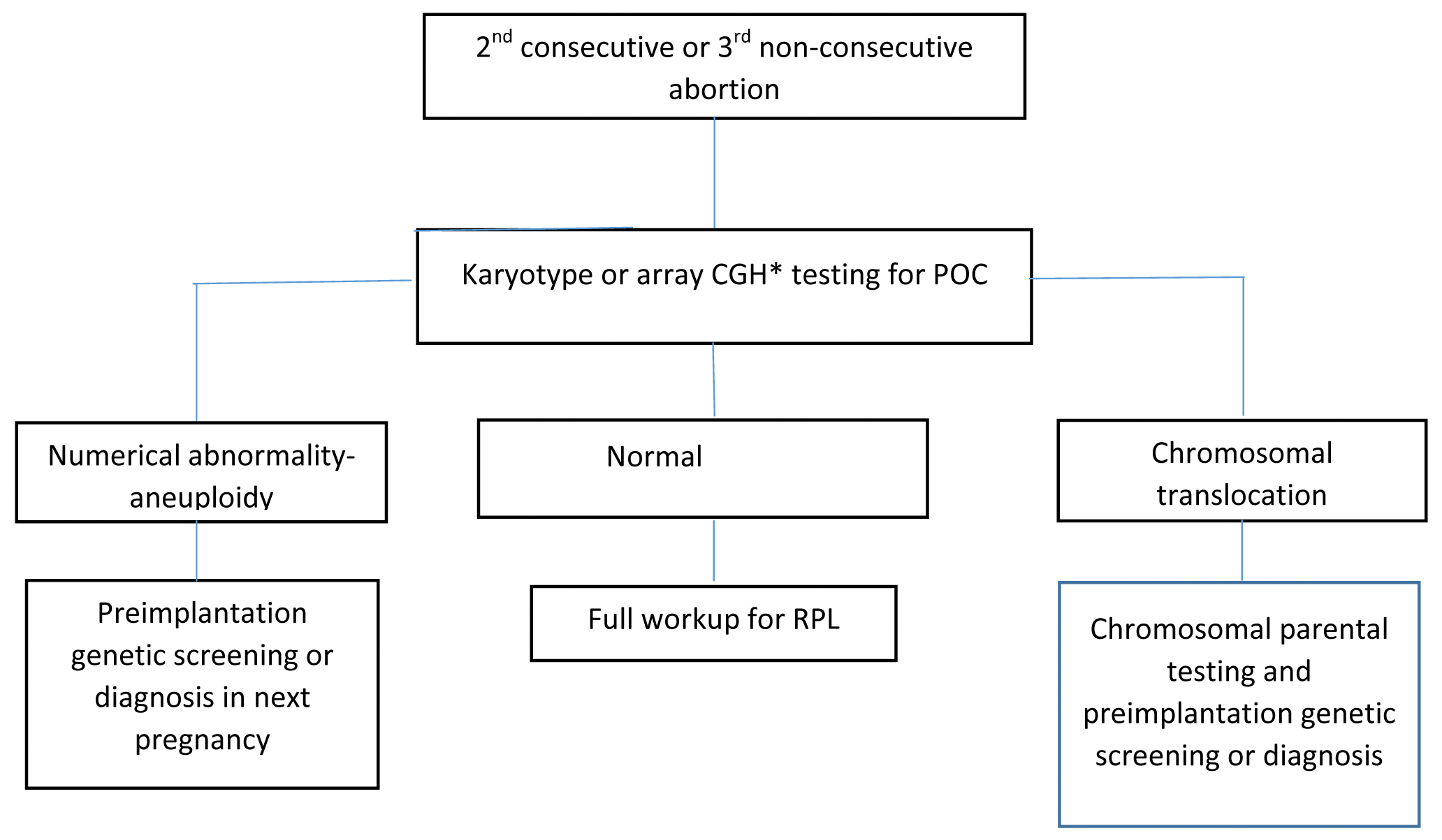
Genetic evaluation: The genetic evaluation of the Product of Conception (POC) of 2nd abortus is recommended in many RPL centres currently, it is shown in [Table/Fig-3] [23]. The use of new techniques, such as single-nucleotide polymorphism microarrays and Comparative Genomic Hybridisation (CGH), has resolved issues with conventional karyotype and allows for a 23-chromosome pair analysis. Array CGH is a currently preferred technique above karyotype and Fluorescent In Situ Hybridisation (FISH). Parental karyotype is ordered only when the chromosomal abnormality (aneuploidy, unbalanced chromosomal translocation or inversion etc.,) is detected in POC. It is reported that such a strategy is more cost-effective than the classic evaluation [6]. However, in the absence of POC karyotype or euploid POC, full maternal workup of RPL is suggested. Parental karyotype is not routinely recommended in couples with RPL. It could be carried out after individual assessment of risk, for instance, strong family history of RPL, offspring with the congenital abnormality or POC with the chromosomal anomaly.
Workup with genetic testing of POC.
POC: Product of conception; CGH: Comparative genomic hybridisation
*can not detect balanced rearrangement
Treatment of RPL: A sympathetic attitude by a physician is very essential. Sincere appreciation of the distress and grief permits thorough discussion with patient and partner. The main concerns of couples are to find the cause and to establish the risk of recurrence. Up to 50% of the couple have unexplained RPL. These couples should be emphasised about excellent pregnancy outcome as average next pregnancy live birth rate is 60-70% with supportive care. Maternal age, the gestational age at which prior losses have occurred, number of previous pregnancy losses, prior live birth and chromosomal constitution of the product of conception have a prognostic role.
1) Genetic Abnormality
Genetic consultation is advised for the chromosomal abnormality found in POC or parental peripheral blood testing. Parents with balanced translocation on cytogenetic testing can have offspring with balanced, unbalanced or totally normal chromosomal makeup. The unbalanced translocations in foetus can result in miscarriage, intrauterine growth restrictions, lethal congenital abnormalities requiring termination of pregnancy, stillbirth and live birth with congenital defects in pregnancies, hence need for prenatal diagnostic procedures should be offered [24]. The risk of abnormal offspring with inversion depends on the size and location of the inversion and the carrier status of gender.
The risk of recurrent aneuploidy is dependent on which parent is heterozygous for the translocation and chromosomes involved. In general, the risk is higher (up to 16%) if the translocation is of maternal origin [24]. All couples with parental chromosomal abnormality should be offered for Pre-Implantation Genetic Testing (PGD) to exclude serious chromosomal abnormality in the embryo, if not feasible, then prenatal diagnosis should be advised. To overcome problems related to imbalance translocations, in vitro fertilisation with PGD-SR (structural rearrangement) is suggested. The possibility of having live birth with abnormal karyotype is very low (<1%), making utilisation of test less frequent. The PGD-A (A-aneuploidy) testing is done at blastomere stage (on one cell) or through polar body testing. For RPL cases, transfer of chromosomally normal embryos have been suggested, however, it has not shown to increase live birth rates and hence, it is not recommended [6]. In vitro fertilisation and use of donor gametes may be suggested for structural rearrangement involving homologous chromosomes, as it precludes live birth.
It is seen that higher the number of miscarriages, less likely the chromosomal abnormalities would be [10]. Thus, the incidence of embryonic numerical chromosomal abnormalities is lower in women with RPL than in those with sporadic miscarriages [25]. Similarly, age related risk of aneuploidy is found to be lower in women with RPL than in those who undergo sporadic miscarriage [25].
2) Uterine Abnormality
Randomise control trial evaluating the effect of surgical treatment of uterine anatomic anomalies on live birth is lacking. However, studies have shown that correction of septate defects have shown to improve pregnancy rates and should be considered (hysteroscopic preferred) [2,13]. Metroplasty is recommended only as a last resort for a bicornuate uterus. No corrective surgery is recommended for unicornuate uterus or uterus didelphys. Asymmetric fusion defects carry worst prognosis and septate, bicornuate and didelphic uteri carry the better prognosis.
Acquired uterine cavity distortion can be dealt with adhesiolysis in cases with endometrial adhesions, removal of submucosal myoma and polyp through hysteroscope is recommended in women with RPL. Lastly, surrogacy may be the option for irreversible uterine abnormalities [12,13]. Few studies have reported that antibiotic improves live birth rates, however, the level of evidence is weak [6].
3) Antiphospholipid Antibody Syndrome
The live birth rate among the pregnancies with antiphospholipid antibody is 10% without any pharmacological treatment. The recommended treatment for APS in pregnancy is low dose aspirin and heparin. It reduces subsequent miscarriage by 54% than aspirin therapy alone (live birth rate 74% with combined therapy versus 42.9% with aspirin alone). Heparin inhibits the antibodies from binding to the trophoblast, prevent complement activation, and promote trophoblastic invasiveness [23].
There are strong evidences to start heparin and aspirin for treatment of proven APLA. Heparin (5,000 IU twice a day) should be started from the confirmation of pregnancy and stopped 12-24 hours before delivery. It is recommended to restart at least 4 hours later of spinal or epidural anaesthesia in postpartum period for 4-6 weeks. Low dose aspirin is started in preconception period and should be stopped 4 weeks before the expected date of delivery, should be restated 24-48 hours postpartum for lifelong [1,23]. Many studies have confirmed that the combined treatment of aspirin and heparin increases the Live Birth Rate (LBR) compared to aspirin alone. [23,26]. Low Molecular Weight Heparin (LMWH) was more recently suggested as an alternative to heparin, with similar efficiency and safety. However, large RCTs comparing the two regimens are still lacking in this specific group of patients [6].
4) Endocrine Abnormality
Treated thyroid dysfunction and/or diabetes improve live birth rates. High prolactin levels should be treated with dopamine agonists. However, till date, only small evidence suggests treating with dopamine agonist drugs to reduce miscarriage in the hyperprolactinemic woman. Evidence for supplementation with progesterone or human chorionic gonadotropin or treatment with metformin for RPL during pregnancy is insufficient. Leutinizing Hormone (LH) suppression does not show an increase in live birth rate among women with RPL and PCOS [23].
5) Others
a) Thrombophilia: Heparin as a therapeutic agent for inherited thrombophilia is not very well studied and due to paucity of evidences, it is not recommended in first trimester RPL, however, few studies have reported its beneficial effect for RPL in second trimester [2].
b) Immunological dysfunction: Immunotherapy with intravenous immunoglobin, paternal cell immunization, and third party donor leukocytes are not recommended for unexplained pregnancy losses [2].
c) Infection, psychological, lifestyle and idiopathic causes: Tender loving care is important component of treatment for psychological factors and unexplained pregnancy losses. Change in lifestyle and infection treatment is recommended when indicated. [2,23].
Conclusion
A workup for possible causes of RPL is recommended in most of the patients after two abortions or following one late second trimester mishap. Proposed evaluation for RPL includes history to get the clues on aetiology, physical examination, and various tests to identify underlying maternal abnormalities. A physician should be aware of unproven tests and controversial treatments to counsel the patient best.
[1]. Brezina PR, Kutteh WH, Classic and cutting-edge strategies for the management of early pregnancy lossObstet Gynecol Clin North Am 2014 41(1):01-18.10.1016/j.ogc.2013.10.01124491981 [Google Scholar] [CrossRef] [PubMed]
[2]. Evaluation and treatment of recurrent pregnancy loss: a committee opinionFertil Steril 2012 98(5):1103-11.10.1016/j.fertnstert.2012.06.04822835448 [Google Scholar] [CrossRef] [PubMed]
[3]. Kolte A, Bernardi L, Christiansen O, Quenby S, Farquharson R, Goddijn M, Terminology for pregnancy loss prior to viability: a consensus statement from the ESHRE early pregnancy special interest groupHuman Reproduction 2014 30(3):495-98.10.1093/humrep/deu29925376455 [Google Scholar] [CrossRef] [PubMed]
[4]. Royal College of Obstetricians and Gynaecologists SAC, Guideline No. 17. The investigation and treatment of couples with recurrent first-trimester and second-trimester miscarriage. 2011; pp. 01-18 [Google Scholar]
[5]. Stirrat GM, Recurrent miscarriageLancet 1990 336(8716):673-75.10.1016/0140-6736(90)92159-F [Google Scholar] [CrossRef]
[6]. El Hachem H, Crepaux V, May-Panloup P, Descamps P, Legendre G, Bouet PE, Recurrent pregnancy loss: current perspectivesInternational Journal of Women’s Health 2017 9:331-45.10.2147/IJWH.S10081728553146 [Google Scholar] [CrossRef] [PubMed]
[7]. Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009Fertil Steril 2009 92(5):1520-24.10.1016/j.fertnstert.2009.09.00919828144 [Google Scholar] [CrossRef] [PubMed]
[8]. Goddjin M, Elson J, Peramo B, Atik RB, Christiansen OB, Kolte AM, Vermeulen N, Recurrent pregnancy lossESHRE 2017 :01-153.Available from: https://www.eshre.eu/Guidelines-and-Legal/Guidelines/Recurrent-pregnancy-loss.aspx [Google Scholar]
[9]. Silver RM, Branch DW, Goldenberg R, Iams JD, Klebanoff MA, Nomenclature for pregnancy outcomes: time for a changeObstetrics & Gynecology 2011 118(6):1402-08.10.1097/AOG.0b013e318239297722105271 [Google Scholar] [CrossRef] [PubMed]
[10]. Werner M, Reh A, Grifo J, Perle MA, Characteristics of chromosomal abnormalities diagnosed after spontaneous abortions in an infertile populationJournal of assisted reproduction and genetics 2012 29(8):817-20.10.1007/s10815-012-9781-322618194 [Google Scholar] [CrossRef] [PubMed]
[11]. Laurino MY, Bennett RL, Saraiya DS, Baumeister L, Doyle DL, Leppig K, Genetic evaluation and counseling of couples with recurrent miscarriage: recommendations of the National Society of Genetic CounselorsJournal of genetic counseling 2005 14(3):165-81.10.1007/s10897-005-3241-515959648 [Google Scholar] [CrossRef] [PubMed]
[12]. Jaslow CR, Carney JL, Kutteh WH, Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy lossesFertility and sterility 2010 93(4):1234-43.10.1016/j.fertnstert.2009.01.16619338986 [Google Scholar] [CrossRef] [PubMed]
[13]. Jaslow CR, Uterine factorsObstetrics and gynecology clinics of North America 2014 41(1):57-86.10.1016/j.ogc.2013.10.00224491984 [Google Scholar] [CrossRef] [PubMed]
[14]. Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P, Clinical implications of uterine malformations and hysteroscopic treatment resultsHuman Reproduction Update 2001 7(2):161-74.10.1093/humupd/7.2.16111284660 [Google Scholar] [CrossRef] [PubMed]
[15]. Jeve YB, Davies W, Evidence-based management of recurrent miscarriagesJournal of Human Reproductive Sciences 2014 7(3):159-69.10.4103/0974-1208.14247510.4103/0974-1208.14247 [Google Scholar] [CrossRef] [PubMed]
[16]. Deans R, Abbott J, Review of intrauterine adhesionsJournal of Minimally Invasive Gynecology 2010 17(5):555-69.10.1016/j.jmig.2010.04.01620656564 [Google Scholar] [CrossRef] [PubMed]
[17]. Regan L, Backos M, Rai R, The Investigation and Treatment of Couples with Recurrent First-Trimester and Second-Trimester MiscarriagesRoyal College of Obstetricians and Gynaecologists Green-top guideline No. 17 2003 LondonRCOG Press:01-13. [Google Scholar]
[18]. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS)J Thromb Haemost 2006 4(2):295-306.10.1111/j.1538-7836.2006.01753.x16420554 [Google Scholar] [CrossRef] [PubMed]
[19]. Kutteh WH, Hinote CD, Antiphospholipid antibody syndromeObstet Gynecol Clin North Am 2014 41(1):113-32.10.1016/j.ogc.2013.10.00424491987 [Google Scholar] [CrossRef] [PubMed]
[20]. Glueck CJ, Wang P, Bornovali S, Goldenberg N, Sieve L, Polycystic ovary syndrome, the G1691A factor V Leiden mutation, and plasminogen activator inhibitor activity: associations with recurrent pregnancy lossMetabolism 2003 52(12):1627-32.10.1016/j.metabol.2003.06.00114669168 [Google Scholar] [CrossRef] [PubMed]
[21]. Stevens SM, Woller SC, Bauer KA, Kasthuri R, Cushman M, Streiff M, Guidance for the evaluation and treatment of hereditary and acquired thrombophiliaJournal of Thrombosis and Thrombolysis 2016 41(1):154-64.10.1007/s11239-015-1316-126780744 [Google Scholar] [CrossRef] [PubMed]
[22]. De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guidelineThe Journal of Clinical Endocrinology & Metabolism 2012 97(8):2543-65.10.1210/jc.2011-280322869843 [Google Scholar] [CrossRef] [PubMed]
[23]. Kutteh WH, Novel strategies for the management of recurrent pregnancy lossSemin Reprod Med 2015 33(3):161-68.10.1055/s-0035-155258626036897 [Google Scholar] [CrossRef] [PubMed]
[24]. Stephenson MD, Sierra S, Reproductive outcomes in recurrent pregnancy loss associated with a parental carrier of a structural chromosome rearrangementHuman Reproduction 2006 21(4):1076-82.10.1093/humrep/dei41716396938 [Google Scholar] [CrossRef] [PubMed]
[25]. Stephenson M, Awartani K, Robinson W, Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control studyHuman Reproduction 2002 17(2):446-51.10.1093/humrep/17.2.44611821293 [Google Scholar] [CrossRef] [PubMed]
[26]. Hoppe B, Burmester GR, Dörner T, Heparin or aspirin or both in the treatment of recurrent abortions in women with antiphospholipid antibody (syndrome)Current Opinion in Rheumatology 2011 23(3):299-304.10.1097/BOR.0b013e328344c3f721346577 [Google Scholar] [CrossRef] [PubMed]