Osteochondroma is a cartilaginous-capped bony outgrowth that is usually located adjacent to the growth plate of long bone [1,2]. In epidemiological studies of bone tumours from different countries including; USA [3], Italy [4], Netherlands [5], Croatia [6], Turkey [7], India [8], Iran [9], China [10], Thailand [11], Nigeria [12], Cuba [13] and Mexico [14], osteochondroma was the most common benign bone tumour affecting children and adolescents.
Osteochondroma may present as a Solitary Osteochondroma (SO), which is more common [3,4,15-19], or as Multiple Osteochondromas (MO). MO or Hereditary Multiple Exostoses (HME) is an autosomal dominant inherited disorder that has been linked to Exostosin (EXT) gene family [1,2,20,21].
Although osteochondroma can arise in any bone, tumours around the knee involving the distal femur and proximal tibia are the most common for both SO and MO [22-28].
Surgery is the main choice of treatment which is usually reserved for symptomatic tumours. Observation is needed for the possibility of secondary malignant transformation. Excision is usually simple but might be complicated by the anatomical consideration of the tumour site. Recurrence is unusual [15-17,26].
Upon review of osteochondroma literature including population-based series, variations of osteochondroma features among different populations were observed. Even, significant variations were noted among studies from within the same population. These variations were noted in different clinical aspects of both SO and MO patients including; sex predilection, familial MO, skeletal distribution, surgical indications, imaging techniques, malignant transformation risk and recurrence rate after resection.
These variations might be related to different population characteristics including genetic variance of different ethnic groups specially for MO [1,2,34-36].
No previous studies were reported describing osteochondroma features in Arab populations from different countries including Jordan. In this study, we reviewed the clinical features of patients with either SO or MO in regard to the age, sex, anatomical location, imaging, surgical management and related complications. This series of Jordanian osteochondroma patients might add to the general knowledge and understanding of this common tumour especially in this population.
Materials and Methods
This was a retrospective study conducted in King Abdullah University Hospital after obtaining approval by the University research committee. Medical records of all osteochondroma patients who had undergone surgery for one or more lesions were reviewed. Only patients with a confirmed histopathological diagnosis of osteochondroma were included . Patients with radiological diagnosis of osteochondroma, who were not operated and without histological confirmation, or detected incidentally, were excluded. The search period extended from March 2004 till June 2017.
The following information was collected from the medical records; age, gender, location, imaging studies, indications for surgery, operative procedure, complications and recurrence.
In order to detect malignant transformation cases, all pathological diagnosis of chondrosarcoma were reviewed to determine if any chondrosarcoma was diagnosed as secondary to osteochondroma. The statistical analysis was performed using student t-test, SPSS version 17 with a significant p-value of less than 0.05.
Results
A total of 109 tumours with a histopathological diagnosis of osteochondroma were identified in 88 patients. Of these, 80% (70/88) had solitary osteochondroma while the remaining 20% (18/88) had multiple osteochondroma with 39 tumours [Table/Fig-1]. Half (9/18) of MO patients had only one tumour resected while the other half had at least 2 tumours resected with a range of 2 to 8 tumours.
Age and sex distribution in solitary and multiple osteochondroma.
| Solitary | Multiple |
|---|
| Number of tumours | 70 | 39 |
| Number of patients | 70 | 18 |
| Males | 47 | 10 |
| Females | 23 | 8 |
| Overall age mean in years (SD) | 19 (8.2) | 14 (7.5) |
| Male age mean in years (SD) | 19 (8.6) | 16 (8.6) |
| Female age mean in years (SD) | 21 (7.5) | 11 (5.2) |
SD: Standard deviation
The 18 patients with MO showed a Male: Female ratio of 1.25:1. The diagnosis of MO was established before the age of 20 years in 89% (16/18) of the patients. The females in this group were diagnosed earlier with an average age of 11 years compared to 16 years for males. In the solitary form, the Male: Female ratio was 2:1. Both males and females in this group were diagnosed around the age of 20 years. However, about two-third (48/70) of these patients were diagnosed before the age of 20 years.
Family history was positive in 50 % (9/18) of MO patients. Two members of three families underwent excision of osteochondroma.; a father and daughter, a brother and sister, and two sisters. The tumours in these families represented about 59% (23/39) of all MO tumours. While 15 tumours were resected in one family, the other two families had only 8 tumours resected.
In MO, 57% of the tumours were in the lower limb with the knee being the most common location [Table/Fig-2]. Proximal humerus, distal radius and distal ulna were equally affected, while tumours of the hand represented 8% of all tumours.
Skeletal distribution of solitary osteochondroma (SO) and multiple osteochondroma (MO).
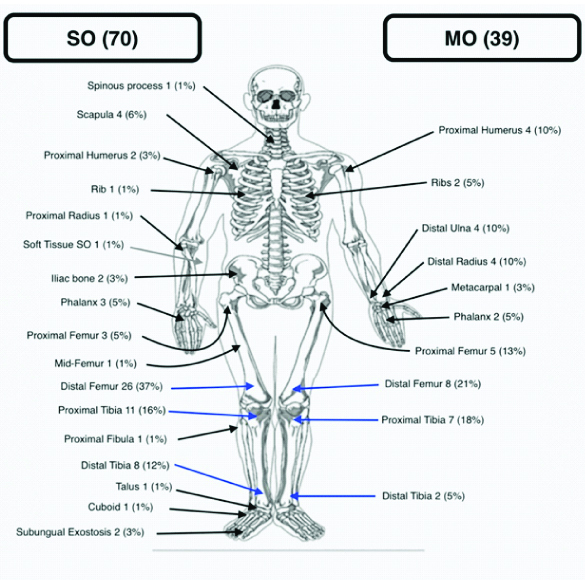
In SO, locations were more variable [Table/Fig-2]. Locations around the knee including the distal femur and proximal tibia constituted about 53% of all solitary lesions. Next common location was the distal tibia followed by the scapula. Soft tissue solitary osteochondroma was diagnosed in one patient with a volar forearm mass.
Conventional radiograph of the affected location in two planes (Anteroposterior and Lateral) was the main diagnostic test. Other imaging modalities including Computed Tomography (CT), Magnetic Resonance Imaging (MRI) and Bone scan were done selectively. MRI and CT were done to evaluate 17 and 14 tumours respectively. Bone scan was performed for six patients with osteochondroma. The indications for these studies included: diagnosis confirmation in inaccessible locations, screening for multiple lesions, pre-operative planning, and clinical suspicion of malignant transformation.
The main indications of surgery for the 109 tumours were; mass (49/109), pain (39/109) and a combination of mass and pain with a decreased range of motion of adjacent joints (16/109). Other less common indications included: bursa (2/109), deformity (2/109) and intermittent painful vascular claudication of the left leg. (1/109).
All patients underwent excision of the tumour. However, corrective osteotomy and internal fixation were needed in two patients to correct the associated deformities. In one MO patient with forearm deformity; excision of the ulnar exostosis and corrective osteotomy of the radius were performed. Also, in one SO patient with Varus ankle deformity, excision of the distal tibia osteochondroma and corrective osteotomy of the distal fibula were performed.
The follow-up period ranged from 1-13 years with an average of 5.6 years. During this time, no recurrence was observed in MO patients. However, recurrence was observed in three SO patients after an average of 33 months after their initial surgery. The recurring tumours were in the little finger middle phalanx, the distal femur and the proximal femur.
The cartilaginous cap thickness was not documented for all tumours on histological examination, however, the cap thickness, in the 63 documented tumours ranged from 1 mm up to 30 mm. None of these patients were found to have malignancy.
Discussion
This series reviews the clinical aspects and management considerations in a group of Jordanian patients with osteochondroma adding to other population-based series from different countries. A summary of the main osteochondroma features from different series is shown in [Table/Fig-3] [15-21,25-27,35-43].
Summary of osteochondroma features from different series [15-21,25-27,35-43].
| Country | Authors | Age* | M/F** | Family***% | Recurrence%. | Malignancy% |
|---|
| SO studies |
| Turkey | Saglik Y et al., [15] | 21.1 | 1.9 | | 6.9 | 2.2 |
| China | Tong K et al., [17] | 21.8 | 1.9 | | 4.6 | 1 |
| Germany | Bottner F et al., [16] | | | | 1.7 | 3.6 |
| Herget GW et al., [19] | | | | 8.3 | 0 |
| Russia | Rogozhin DV [18] | | 1.6 | | | 1 |
| France | Essadki B [25] | 21 | 1.37 | | | |
| Spain | Florez B et al., [26] | | | | 5.3 | 1.7 |
| USA | Humbert ET et al., [37] | | | | 1.8 | |
| Jordan | Current study | 19 | 2 | | 4.3 | 0 |
| MO studies |
| Turkey | Saglik Y et al., [15] | 21.3 | 2.45 | 62.3 | 23.2 | 11.6 |
| China | Tong K et al., [17] | 16.9 | 2.92 | 14.7 | 19.6 | 2.9 |
| Guo XL et al., [38] | | 1.5 | 96 | | 2 |
| Germany | Bottner F et al., [16] | | | | 13.3 | 3.3 |
| Herget GW et al., [19]c | | | | 45 | 0 |
| Jager M et al., [39] | 4 | 1.7 | | 6.3 | 3.8 |
| Heinritz W et al., [40] | | 2.3 | 65 | | |
| Russia | Rogozhin DV [18] | | 1.6 | | | 1% |
| USA | Pierz KA et al., [27] | 5.75 | 1.1 | 46.5 | | 0 |
| Schmale G et al., [41] | 3 | 1.5 | 90 | 2.7 | 0.9 |
| Japan | Ishimaru D [35] | | 1.3 | | | |
| UK | Porter DE et al., [21] | | 0.9 | | | 4 |
| Brazil | Santos SCL et al., [42] | 20 | 1.22 | 63 | | 6 |
| France | Legeai-Mallet L et al., [20]we found 109 familial forms (62%) | | 1.4 | 62 | | 0.57 |
| Spain | Sarrion P et al., [36] | 2 | 1.6 | | | 2.5 |
| Bulgaria | Stancheva-Ivanova MK et al., [43] hereditary multiple osteochondroma (MO) | | 0.9 | 65 | | 0 |
| Russia | Rogozhin DV [18] | | 1.8 | | | 5 |
| Jordan | Current study | 14 | 1.25 | 50 | 0 | 0 |
*Age (years); **M/F: Male/Female ratio; ***Family history; %: Percentage
In this series, SO was four times more common than MO. Published data revealed a variable range of SO:MO ratio from 2:1 as reported by Bottner F et al., [16] from Germany to more than 4.5:1 in a series from Turkey [15].
Male predominance was documented in both SO and MO group in this series. While many authors [15,17,18,20,25,27,35,36,38-42] reported a similar male predominance, some series from UK [21] and Bulgaria [43] revealed a slight female predominance in MO patients.
The reported average age varies widely. Some studies [27,36,39,41] reported a young average age since they reported their patients with MO at the time of diagnosis . However, other studies [15] reported an older average age as patients were reported at the time of surgical treatment as the case in this series.
Family history in MO patients is variable even between studies from the same country [17,27,38,41]. In this study, family history was present in half of the patients which is similar to that reported by Pierz KA et al., [27]. However, many other studies from different countries revealed a higher prevalence of familial MO [Table/Fig-2]. This reflects on the genetic nature of this disease which has been associated with mutations in Exostosin (EXT) tumour suppressor gene family. These mutations might be variable among different populations [1,20,35,36].
The involvement of different skeletal locations by SO is variable. In this series, more than half of the tumours occurred around the knee which is little more than reported by Saglik Y et al., [15] from Turkey and Tong K et al., [17] from south China who reported knee involvement in 40% and 50% of their patients, respectively [15,17]. Both authors also reported the humerus as the third most common bone involved in contrast to this series in which the scapula was slightly more affected.
Unusually reported locations included; the mid-shaft femur, proximal radius, cuboid, talus, spinous process and ribs [17,28,38,39,44-46]. Isolated SO located in the soft tissue is a rare occurrence of osteochondroma [47]. One patient in this series presented with a hard-soft tissue mass in the volar aspect of mid-forearm that was proven histopathologically as osteochondroma.
Different series of MO report variable rate of occurrence in different locations. Similar to this series, the knee was the most common location in most published series from different countries including USA [27,41], China [17], Germany [39], UK [21,28], Japan [48], and Turkey [15].
Upon review of different pre-operative imaging modalities, conventional radiography was the standard initial test for all patients and in two-third of the tumours was the only study needed prior to surgery. This may be related to the ease of diagnosis of osteochondroma in the majority of cases based on x-ray alone by observing the continuity of the lesion with the surface of the bone which is pathognomonic for osteochondroma [24,49]. MRI, CT and bone scan were done selectively. Although, we performed MRI slightly more than CT in this series, Saglik Y et al., [15] from Turkey reported more CT scans than MRI scans to evaluate their patients [15]. This might reflect on the variation among the treating physicians and institutions in choosing the best imaging modality in the management and follow-up of osteochondroma patients.
Indications for surgery in this series are similar to those reported in different published articles [2,16,17,22,24,26,49-52]. In 45% of osteochondroma tumours, enlargement of the mass causing cosmetic concerns and interference with adjacent joint motion was the main indication for surgery. Pain was another indication in about 36% of osteochondromas. The pain was usually localised to the tumour with radiation along the distribution of adjacent nerves in some occasions.
Among other indications for surgery was bursa formation which was detected overlying osteochondromas tumours that involved the scapula and the fourth rib. Localisation in these superficial anatomical sites might be a risk factor. This rare manifestation of this tumour can mimic malignant transformation both clinically and radiologically [53,54].
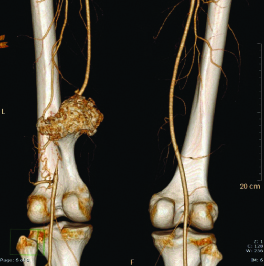
Vascular complications of osteochondroma, which might be associated with significant morbidity, are rare indications for surgery [50]. In one patient who presented with painful intermittent vascular claudication of his left leg, large distal femur SO was found to be obliterating a significant segment of the superficial femoral artery [Table/Fig-4].
Distal femur osteochondroma with obliteration of the superficial femoral artery.
Although resection of osteochondroma is usually a straight forward procedure, the anatomical location and tumour size might impose an added challenge. For example, in the proximal femur, different surgical approaches had been described for optimal resection in this area without jeopardising the vascularity of the femoral head or increasing the risk of subsequent fractures [55,56].
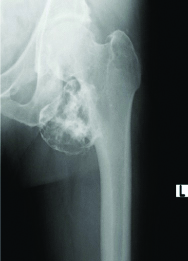
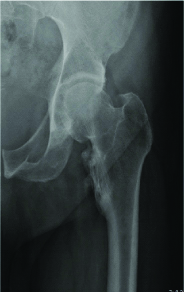
We used anterior, medial or lateral hip surgical approaches for proximal femur Osteochondroma excision without adding any internal fixation devices [Table/Fig-5,6]. None of these patients developed avascular femoral head necrosis or posto-perative fractures, however, one patient had a recurrence which was treated by re-excision. This might be due to the more conservative resection in this location given the benign nature of osteochondroma.
Solitary Osteochondroma of the proximal femur X-ray (pre-operative).
Post-operative X-ray after resection of proximal femur Osteochondroma.
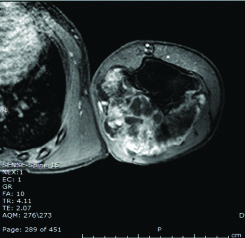
Another challenging location is the proximal humerus specially with large tumour size and postero-medial involvement [57]. One patient presented with large osteochondroma filling his axilla [Table/Fig-7]. Through posteromedial surgical approach, resection of most of the tumour was achieved with no neurovascular complications or recurrence over his two years follow-up period.
Axial MRI of the left proximal humerus Osteochondroma showing posteromedial involvement.
Recurrence rate after osteochondroma resection is variable in literature with higher recurrence in MO. However, in this series; no recurrence was documented in MO patients while 4.3% of SO patients developed recurrence after resection.
Of note was that none of the patients developed post-resection fracture although no prophylactic internal fixation metal was used. This might be explained by the more conservative resection given the benign nature of osteochondroma and the low risk of recurrence.
Risk of malignant transformation reported by population-based studies is variable with higher risk in MO [Table/Fig-3]. None of SO or MO patients developed malignant transformation over the 13 years search period. This might be related to the low risk of malignant transformation [31-33], in addition to the relatively small number of the patients in this series specially MO patients who have higher risk of malignant transformation.
Different variables were used to describe the severity of MO including deformities requiring corrective osteotomy in addition to the number of osteochondromas in each patient. In this series, the average number of resected tumours per MO patient of 2.2 was significantly less than that reported by Saglik Y et al., Pierz KA et al., and Jager M et al., who reported an average of 3.3, 3.4 and 5.6 respectively (p-value=0.01) [15,27,39]. In addition, corrective osteotomies, performed in 5.6% of patients, were significantly less frequent when compared to other series by Saglik Y et al., Tong K et al., and Pierz KA et al., who reported corrective osteotomies in 24.6%, 7.8% and 14% of their patients respectively (p-value=0.025) [15,17,27]. This can indicate a milder form of MO in this group of Jordanian population.
Limitation
The relatively small number of patients specially in the MO group is the main limitation. In addition, this study was conducted on selected patients referred for surgical treatment. Although this might reflect on the more clinically significant tumour, it might have excluded patients with milder and less symptomatic tumour.
Conclusion
SO was more common than MO. In both groups, most patients were diagnosed before the age of 20 years and males were more affected although slightly in MO as compared to females. Family history was positive in half of the MO patients. Tumours around the knee were the most common in both groups. Common indications for surgery included mass and pain. Rare indications included; vascular claudication, soft tissue osteochondroma and bursa formation. Recurrence was observed only in SO while no secondary chondrosarcoma was documented in either group. Compared to other populations, Jordanian MO patients presented a milder form of MO with low number of tumours in each patient and low risk of associated deformities. Given the low risk of recurrence, post- resection fracture and secondary malignant transformation in this series, we recommend a conservative surgical resection approach to minimise possible complications especially in anatomically difficult locations. Also, MRI can be limited for assessment of malignant transformation and pre-operative planning specially in unusual locations. Further genetic studies in this population may help in explaining the clinical variation of this disease.
SD: Standard deviation
*Age (years); **M/F: Male/Female ratio; ***Family history; %: Percentage