Deep vein thrombosis and pulmonary embolism forms Venous Thromboembolism (VTE), a potentially life threatening disease caused by multiple interactions between different inherited and environmental factors. The environmental risk factors include prolonged immobility, surgery, malignant diseases, heart disease, pregnancy, hormone therapy, and oral contraceptives, and are mostly identified in young and middle-aged individuals [1]. Although, VTE is uncommon among individuals younger than 40 years of age, it affects more females than males in this age group [2], possibly because of the presence of risk factors, such as pregnancy and the use of oral contraceptives [3]. By contrast, the incidence of VTE in older adults (>40 years of age) is similar in both males and females [2] and affects 1-2 adults per 1000, annually [4]. Therefore, it is important to identify individuals at risk and to offer them adequate treatment and/or prophylaxis [5].
Among the genetic risk factors, mutation in Factor V gene is the most common mutation underlying hereditary thrombophilia in Western countries [6]. Factor V Leiden variant is a mutant form of factor V that is common among Caucasians and Middle-Easterners and moderately prevalent among Hispanic Americans, Indians, and Africans [7,8]. The factor V Leiden mutation renders activated Factor V (FVa) resistant to cleavage by Activated Protein C (APC), which results in an increased rate of thrombin generation and higher levels of prothrombin fragment 1+2 [9-11]. This mutation confers a 5 to 10-fold greater risk of DVT among heterozygous individuals and a 50 to 100-fold greater risk among homozygotes compared with normal individuals [12]. Factor V Leiden is identified in approximately 20% of patients diagnosed with VTE. Genetic testing for Factor V Leiden is used in cases showing equivocal results of a modified APC resistance-screening assay to provide appropriate clinical management [13].
The second most common genetic risk factor for hereditary thrombophilia is the G to A substitution at nucleotide position 20210 in the 3’UTR of the prothrombin gene, which leads to an increase in prothrombin production and 3-fold greater risk of DVT [8,14]. Prothrombin 20210G>A mutation is an autosomal dominant trait. It has the highest prevalence among Caucasians of European origin, with a carrier frequency of 1-3% in the general population and approximately 6% of VTE and in 10% in patients with thrombophilic families [10].
Single Nucleotide Polymorphisms (SNPs) in factor V and prothrombin genes are frequently co-inherited [11] and are genotyped via (PCR-RFLP) analysis. Risk for VTE is higher in individuals carrying both gene mutations compared with those carrying one of these gene mutations [15]. However, whether these gene mutations cause mortality among African populations, especially the Sudanese, remains controversial [16-18]. Moreover, the role of environmental risk factors in the development of DVT in the Sudanese population is poorly characterised.
The purpose of the study was to determine the prevalence of the two mutations in Sudanese DVT patients, and to investigate the role of environmental risk factors in the manifestation of DVT.
Materials and Methods
Study Group
This case-control study was conducted at the Sudan University of Science and Technology research laboratory from July 2014 to July 2017 in Khartoum, Sudan. A total of 192 Sudanese individuals were recruited for this study, including 100 patients (75 females and 25 males) with a confirmed DVT diagnosis based on the results of duplex ultrasound and 92 healthy controls (61 females and 31 males). The age range of patients was 19-90 years, while that of controls was 18-71 years. The mean age±Standard Deviation (SD) of the patients and controls was 41.60±17.28 years and 31.65±10.08, years respectively.
Inclusion criteria: The study included only adult duplex ultrasonography DVT diagnostic patients who accepted to participate and who demonstrated positive D dimer test.
Exclusion criteria: Subjects who were younger than 18 years or who refused to participate were excluded.
Subjects in the control group had no previous history of VTE or other coagulation disorders. The demographic and clinical data of all participants were collected using a standardised questionnaire. Obesity was defined as a Body Mass Index (BMI) of 30 kg/m2 or above. Physician’s assessment was accepted in cases where weight and height data were unavailable. EDTA-blood and citrated blood samples were collected from each individual for DNA extraction and coagulation tests, respectively. Informed consent was obtained from all the subjects.
Ethical Approval
This study was conducted in compliance with relevant national regulations, institutional policies, and the tenets of the Helsinki Declaration and was approved by the Research Ethical Committee of Sudan University of Science and Technology [R#2014/7/7].
DNA Extraction
Genomic DNA was extracted from peripheral blood using the G-spin DNA extraction kit (iNtRON Biotechnology Inc., Jungwon-gu, South Korea) according to the manufacturer’s protocol.
Coagulation Assays
Coagulation tests, including Prothrombin Time (PT) and activated partial thromboplastin time (APTT) were performed using STart 4 Haemostasis Analyser (Diagnostica Stago, Asnieres, France). Fibrinogen assays and D-dimer tests were performed using Multifibren U and Innovance D-dimer reagents, respectively, with BCS XP System (Siemens Healthcare GmbH, Erlangen, Germany). The APC resistance test (APC-R) was performed using STA-STACLOT (Diagnostica Stago, Asnieres, France). Sandwich Enzyme-linked Immunosorbent Assay (ELISA) was performed for the quantitative detection of prothrombin fragment 1+2 using Human Prothrombin Fragment 1+2 ELISA Kit (Abbexa, Cambridge Science Park, UK).
Factor V Leiden Genotyping
Genotyping for Factor V Leiden was performed using PCR-RFLP analysis with HindIII restriction endonuclease. A 241 bp fragment encompassing the 1691G>A substitution of factor V exon 10 was PCR amplified as previously described using the forward primer 5’-TCAGGCAGGAACAACACCAT-3’ and reverse primer 5’-GGTTACTTCAAGGACAAAATACCTGTAAAGCT-3 [19]. The expected banding patterns of digested PCR products were a 241 bp fragment for the wild-type allele, 209 bp and 32 bp fragments for factor V Leiden homozygotes, and all three molecular weight bands for factor V Leiden heterozygotes.
Prothrombin 20210G>A Genotyping
Prothrombin gene polymorphism was detected according to method described by Poort SR et al., [8]. Forward and reverse primers used for exon 14 were 5’-TCTAGAAACAGTTGCCTGGC-3’ and 5’-ATAGCACTGGGAGCATTGAAGC-3’, respectively. For RFLP analysis, PCR products were digested with HindIII. The expected banding patterns were a 345 bp fragment for the wild-type allele, 322 bp and 32 bp fragments for homozygous mutants, and both molecular weight bands for heterozygous mutant.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (IBM SPSS Statistics version 20.0; Armonk, NY, USA). Significant differences in continuous variables were analysed using the Student’s t-test. Results were expressed as mean±SD. A p-value less than 0.05 were considered as statistically significant.
Results
In the present study, we evaluated 100 patients (25 males, 75 females) with proven DVT diagnosis and 92 healthy controls (31 males, 61 females). No significant differences in the prevalence of DVT were detected between males and females (p=0.185). Among the 100 patients studied, DVT was the most prevalent at age 18 to 45 years in both males (n=16; 22.9%) and females (n=54; 77.1%).
The 100 DVT patients were divided into three age groups (18-45 years, 46-65 years, and 66-90 years) and assessed for the environmental risk factors that are generally known to predispose them to DVT [Table/Fig-1]. Risk factors, namely, immobility status and cardiovascular disease were the most significantly associated with age (p<0.0001). Previous history of DVT was found in 73.3% of patients in the age group of 66-90 years (n=15). About 93.3% of the DVTs were localised in the left leg and 6.7% in the right leg. Additionally, 88% of the DVTs were proximal and 12% were distal. Proximal DVT was significantly associated with age in all age groups (p<0.0001).
Demographic and clinical characteristics of DVT patients categorised in three age groups.
| Charateristics | Age group |
|---|
| 18-45 years | 46-65 years | 66-90 years |
|---|
| F | % | F | % | F | % | p-valuea |
|---|
| Gender | Male | 16 | 22.9 | 3 | 20.0 | 6 | 40.0 | 0.368 |
| Female | 54 | 77.1 | 12 | 80.0 | 9 | 60.0 |
| Diagnosis | Proximal DVT | 70 | 100.0 | 10 | 66.7 | 8 | 53.3 | <0.0001* |
| Distal DVT | 0 | 0.0 | 5 | 33.3 | 7 | 46.7 |
| Previous history of DVT | Yes | 0 | 0.0 | 2 | 13.3 | 11 | 73.3 | <0.0001* |
| No | 70 | 100.0 | 13 | 86.7 | 4 | 26.7 |
| Affected leg | Right leg | 6 | 8.6 | 0 | 0.0 | 1 | 6.7 | 0.830 |
| Left leg | 64 | 91.4 | 15 | 100.0 | 14 | 93.3 |
| Obesity | Yes | 5 | 7.1 | 1 | 6.7 | 0 | 0.0 | 0.827 |
| No | 65 | 92.9 | 14 | 93.3 | 15 | 100.0 |
| Hormonal Therapy | No | 70 | 100.0 | 15 | 100.0 | 15 | 100.0 | NA |
| Oral Contraceptives | Yes | 19 | 35.2 | 2 | 16.7 | 0 | 0.0 | 0.059 |
| No | 35 | 64.8 | 10 | 83.3 | 9 | 100.0 |
| Cigarette Smoking | Yes | 10 | 14.3 | 2 | 13.3 | 0 | 0.0 | 0.389 |
| No | 60 | 85.7 | 13 | 86.7 | 15 | 100.0 |
| Under Surgery | Yes | 30 | 42.9 | 3 | 20.0 | 4 | 26.7 | 0.167 |
| No | 40 | 57.1 | 12 | 80.0 | 11 | 73.3 |
| Bone Fracture | Yes | 3 | 4.3 | 0 | 0.0 | 0 | 0.0 | 1.000 |
| No | 67 | 95.7 | 15 | 100.0 | 15 | 100.0 |
| Immobility Status | Yes | 5 | 7.1 | 0 | 0.0 | 10 | 66.7 | <0.0001* |
| No | 65 | 92.9 | 15 | 100.0 | 5 | 33.3 |
| Cardiovascular Disease | Yes | 2 | 2.9 | 4 | 26.7 | 12 | 80.0 | <0.0001* |
| No | 68 | 97.1 | 11 | 73.3 | 3 | 20.0 |
| Renal Failure | Yes | 2 | 2.9 | 1 | 6.7 | 0 | 0.0 | 0.661 |
| No | 68 | 97.1 | 14 | 93.3 | 15 | 100.0 |
| Cancer | Yes | 3 | 4.3 | 0 | 0.0 | 1 | 6.7 | 0.766 |
| No | 67 | 95.7 | 15 | 100.0 | 14 | 93.3 |
| Anticoagulant therapy | Heparin | 44 | 62.9 | 8 | 53.3 | 1 | 6.7 | 1.000 |
| Warfarin | 0 | 0.0 | 0 | 0.0 | 2 | 13.3 |
| Heparin and Warfarin | 26 | 37.1 | 7 | 46.7 | 12 | 80.0 |
| Factor V | Homozygous wild-type (G/G) | 70 | 100.0 | 15 | 100.0 | 15 | 100.0 | NA |
| Prothrombin gene | Homozygous wild-type (G/G) | 70 | 100.0 | 15 | 100.0 | 15 | 100.0 | NA |
ap-values were determined using Student’s t-test; NA: Not applicable; F: Frequency; *significant
Significant differences were observed between DVT patients and healthy controls in the levels of prothrombin fragment 1+2, PT, APTT, and D-dimer (p<0.05) [Table/Fig-2]. None of the 100 DVT patients tested positive for factor V Leiden mutation [Table/Fig-3] or the prothrombin 20210G>A mutation [Table/Fig-4]. All 92 healthy controls were negative for both these mutations. As a result, no correlation against the risk factors was possible.
Summary of coagulation characteristics of blood samples obtained from 100 Deep Vein Thrombosis (DVT) patients and 92 healthy controls of Sudanese origin.
| Coagulation characteristics | Subjects | Mean±SD | p-valuea |
|---|
| Prothrombin Time (s) | Patients | 23.36±9.62 | <0.0001* |
| Controls | 11.41±0.83 |
| Activated Partial Thromboplastin Time (s) | Patients | 41.82±13.59 | <0.0001* |
| Controls | 31.05±6.08 |
| D-dimer (mg/L) | Patients | 0.67±0.20 | 0.044 |
| Controls | 0.30±0.16 |
| Prothrombin Fragment 1+2 (ng/mL) | Patients | 12.38±1.32 | <0.0001* |
| Controls | 4.99±2.44 |
| Activated Protein C resistance (Ratio) | Patients | 2.66±0.30 | 0.850 |
| Controls | 2.67±0.34 |
| Fibrinogen (gm/L) | Patients | 2.93±0.46 | 0.766 |
| Control | 2.90±0.49 |
aStatistical significance of the differences between patients and controls were determined using Student’s t-test; *significant
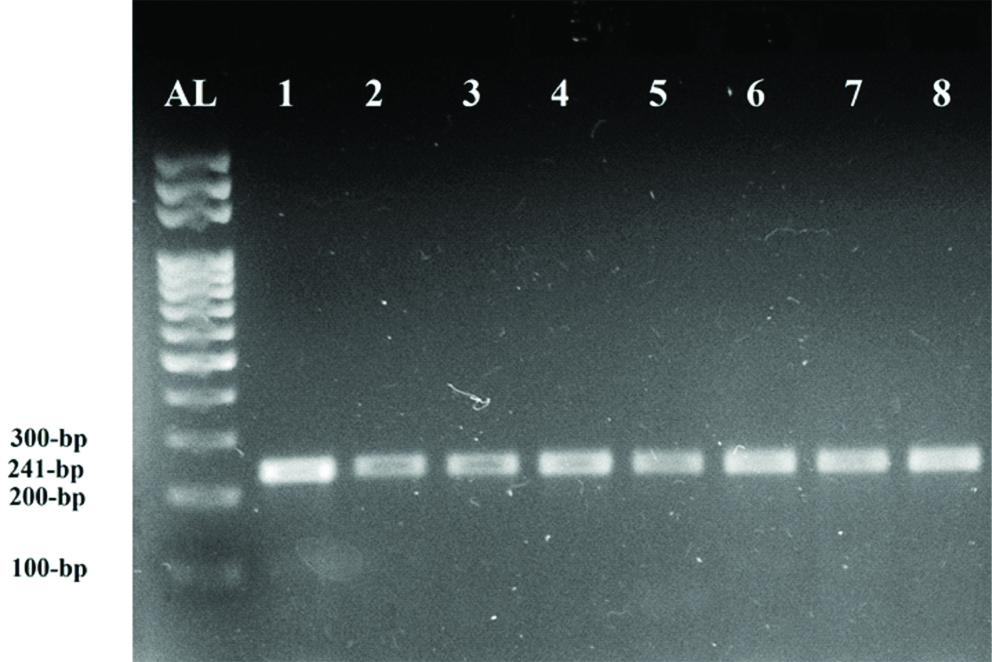
Ethidium bromide-stained gel image showing PCR-RFLP analysis of seven representative samples for detecting factor V Leiden mutation. All samples carried the wild-type allele of factor V, indicated by the 241bp band. AL: 100 bp Allelic leader, 1: undigested PCR product (241 bp), and 2-8: PCR products digested with HindIII.
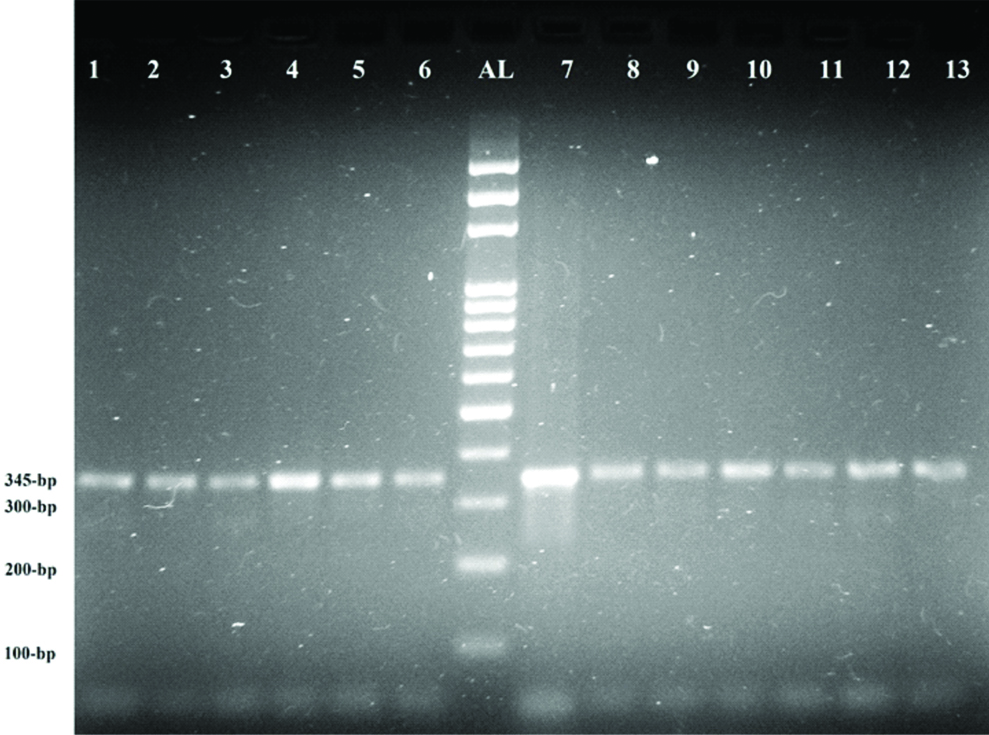
Ethidium bromide-stained gel image showing PCR-RFLP analysis of twelve representative samples for detecting prothrombin 20210G>A mutation. All samples carried the wild-type allele of prothrombin, indicated by the 345 bp band. AL: 100 bp Allelic leader, 7: undigested PCR product (345 bp) amplicon, 1-6 and 8–13: PCR products digested with HindIII.
Discussion
Deep vein thrombosis is the most common clinical manifestation of venous thromboembolism and a common cause of death and disability in developing countries [20]. The occurrence of DVT involves the interaction of multiple inherited factors and different modifiable and non-modifiable environmental influential factors [4,21]. The determination of these risk factors heavily influences the prevention strategies of local healthcare agencies. Among the genetic causes of DVT, Factor V Leiden and prothrombin 20210G>A mutations are the most common [22]. Carriers of these polymorphisms are prone to spontaneous or secondary venous thrombotic events at any age [23]. The main environmental risk factors for DVT include old age, obesity, history of VTE, cancer, bed rest for more than five days, surgery, congestive heart failure, bone fracture, hormone therapy, and pregnancy. The risk of DVT increases several folds when both genetic and environmental factors co-exist [24].
In the present study, we evaluated a total of 192 individuals of Sudanese origin, out of which 100 were DVT patients and 92 were healthy controls. None of the 192 individuals carried Factor V Leiden or prothrombin 20210G>A mutations, indicating the absence of a potential genetic drift due to founder effect that may be present in individuals of European origin [25,26]. Moreover, these data also showed that neither one of these gene mutations were associated with DVT in individuals of Sudanese origin, which is consistent with a previous study on an African population [18]. Similarly, Factor V Leiden has been shown to be absent in 1600 individuals from Africa, Southeast Asia, Australasia, and the Americas [27]. The present study results were consistent with a previous study reported that factor V Leiden or prothrombin 20210G>A mutations were not associated with thrombophilia [28]. Factor V Leiden or prothrombin G20210A mutations were absent in DVT cases studied in Chinese population [29]. Moreover, Prothrombin gene mutation as risk for inherited thrombophlia was absent or occurs in a very low frequency in both thrombophilic patients and healthy controls of most South Asian populations [30].
However, a previous meta-analysis has shown that the Factor V Leiden mutation is associated with the risk of DVT in other populations [31]. Also, present study findings were in contrast with a previous study in Bosnia and Herzegovina, where Factor V Leiden and prothrombin 20210G>A mutations were associated with DVT in Bosnian population [32].
In agreement with the results reported by Satoshi O et al., significantly higher levels of plasma D-dimer and prothrombin fragment 1+2 were detected in DVT patients than those in healthy controls [33]. No significant differences were observed in the prevalence of DVT between male and female patients in this study in comparison with healthy subjects. However, among younger adults (≤45 years of age), DVT was more prevalent in females than in males, which is consistent with previously published data [4,34].
The present study data showed that 13% of DVT patients, most of who were in the age group of 66-90 years had previously had single or recurrent episodes of DVT. This is consistent with the results of Anderson FA and Spencer FA showing that the previous history of VTE is significantly associated with increased risk of DVT [24]. Additionally, immobility and cardiovascular disease were found to be significantly associated with the occurrence of DVT among the elderly, which is in agreement with the results of Engbers MJ et al., [35]. A significant association between the use of oral contraceptives and DVT development in female patients in the age group from 18-45 years was identified in present study, this was in the same line with previous studies on the increased risk of VTE and oral contraceptives usage in young females [36]. The present study findings of other environmental risk factors were consistent with previous studies [24], except for obesity (6%), which maybe because of dietary and behavioral differences. The present study confirms the findings of previous studies that have reported the predominance of left-sided DVT [37]. The reason behind the left-side predominance is unknown. Previous studies proposed that left-sided DVTs may be related to compression of the left common iliac vein by the right common iliac artery [37,38]. Additionally, we observed that in most of the cases, DVTs were proximal. This was in complete agreement with a previous study [39].
Limitation
Nonetheless, there were certain limitations in this study. Specifically, the relatively small sample size was used. It is recommended that the future studies be performed with larger sample size which covers all the states of Sudan. Moreover, further investigations into other possible causes of hereditary thrombophilia are needed, such as 677 (C>T) Methylene Tetrahydrofolate Reductase (MTHFR) mutation.
Conclusion
In conclusion, although factor V Leiden and prothrombin 20210G>A mutations are considered as genetic risk factors for DVT, these mutations were not associated with DVT in the Sudanese population examined in present study.
ap-values were determined using Student’s t-test; NA: Not applicable; F: Frequency; *significant
aStatistical significance of the differences between patients and controls were determined using Student’s t-test; *significant