Chronic lower limb swelling is frequently encountered in clinical practice. Secondary lymphoedema and chronic venous insufficiency are two frequent causes of chronic lower limb swelling. The most common form of secondary lymphoedema worldwide is filariasis, due to Wuchereria bancrofti. Chronic inflammation and fibrosis are histological hallmarks of lymphoedema. Chronic venous insufficiency refers to venous dilation and venous reflux of long duration. The classical signs include minimal superficial dilation with associated skin changes and ulceration. Both the conditions mimic each other although the treatment is different. The diagnosis need to be confirmed prior to institution of treatment. We present below two cases of chronic lower limb swelling with a review of literature.
Bancroftian filariasis, Immune response, Lymphoedema, Venous reflux, White blood cells
Case Report
Case 1
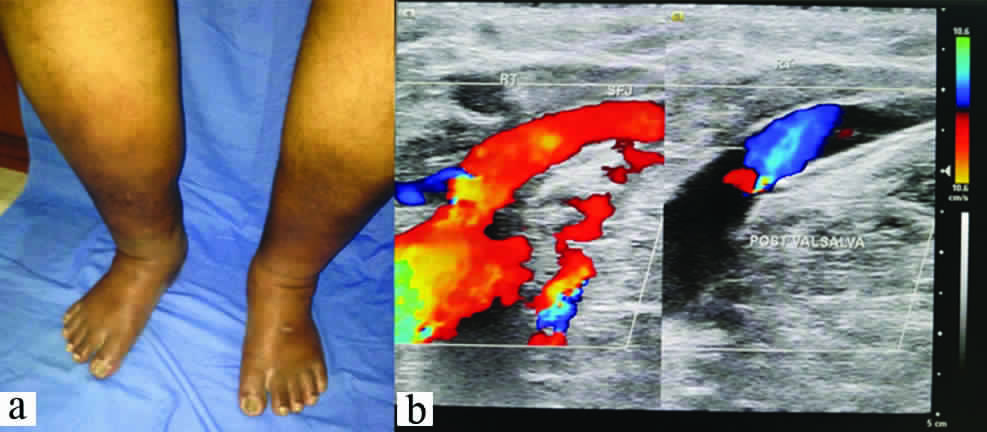
A 75-year-old man, diabetic since last six years presented with oedema, pigmentation and inflammation of both feet, ulcer of about 1.5 cm diameter over right medial malleolus with high grade fever (>103°F) since last three weeks [Table/Fig-1a]. He was under oral hypoglycaemic treatment. A suspicion of inflammation secondary to stasis in chronic venous insufficiency was made. Since the patient hailed from a filarial endemic zone, an OG4C3 test for detecting adult filarial antigen from the blood was done but tested negative. The diagnosis was confirmed by duplex ultrasound which identified abnormal venous reflux [Table/Fig-1b]. Bilateral sapheno-femoral junction incompetence was noted with reflux of venous flow in to the Great Saphenous Vein (GSV) up to knee level. Multiple varicosities were noted in both lower thigh and legs with diffuse subcutaneous oedema in lower third of both legs. An incompetent perforator in right GSV, 15 cm above ankle was visualized. The patient had been treated with a combination of Ciprofloxacin, Ornidazole and Linezolid initially without much improvement. Based on clinical findings, a course of Amoxycillin with Clavulanic acid for two weeks and Dabigatran was started at 150 mg twice a day and continued for four weeks. Insulin was administered as the blood sugar levels were high. Crepe bandage application to lower limb and limb elevation was done. The swelling, redness, pain and fever of the foot reduced to much extent. Over a period of 12 weeks, the pigmentation gradually disappeared. The insulin was discontinued as the sugar levels came to normal and infection subsided. However, the patient was advised to continue oral hypoglycaemics. A small ulcer which was developing in the medial side of ankle due to the stasis of blood, healed and the overlying skin regained its luster. The patient was advised to use elastic compression stockings regularly.
(a) Clinical picture demonstrating pigmentary changes, oedema and inflammation of bilateral lower limbs; (b) Venous doppler showing incompetence at the sapheno femoral junction.
Case 2
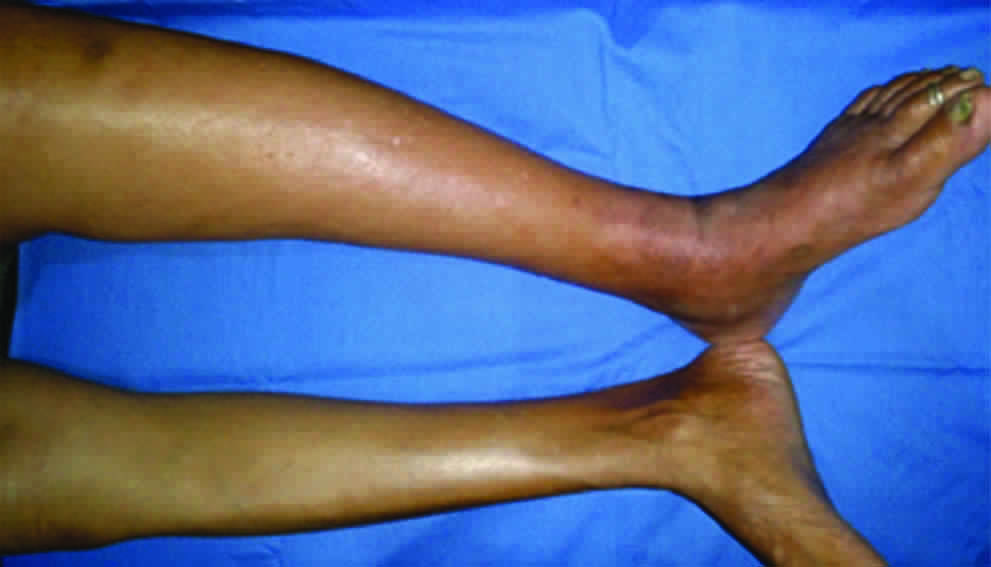
A 53-year-old female reported with a painful, swollen left leg [Table/Fig-2]. She was unable to walk, even raise the leg because of pain since last three weeks. She was not a diabetic or hypertensive. The patient had a long history of filarial lymphoedema with recurrent fever. She belonged to an endemic zone of filariasis with a family history of the disease. On examination, multiple oozing blisters, increased temperature, tenderness and a pitting oedema was noted on the left leg. The left inguinal gland was swollen and tender. The web spaces of the foot were unhygienic and affected by candidiasis. The disease was confirmed by lymphoscintigraphy and a positive OG4C3 test detecting adult filarial antigen from the blood. A diagnosis of filarial dermato-adeno-lymphangitis was made. Antibiotics (4th generation Cephalosporin), analgesics and a course of Diethyl Carbamazine (DEC) for 21 days was started. The patient showed a rapid response within a week. She was advised to complete the course of DEC along with topical anti-fungal treatment and foot hygiene. Crepe bandage application and limb elevation was advised.
Clinical picture demonstrating dermatolymphangitis of left lower limb with oedema, pigmentation and inflammatory changes.
Discussion
Management of chronic swelling of the limbs is a continuing challenge to patients and clinicians. The lymphatic system plays an important role for normal immune function in health and disease. There is no established cure for lymphoedema due to its nature of abnormalities. Hopefully, new ideas will develop to improve accuracy of diagnosis and effective medical and surgical care. Erysipelas is a subnormal inflammation associated with Streptococcus pyogenes [1]. It is often of sudden onset, marked by fever >38°C, chills and general malaise. Local signs develop within a few hours: a red, warm, painful, inflammatory spreading lesion within a few days. Lymphoedema generally does not cause chronic skin ulcers. It is complicated by infection of the skin and deep tissues in 50% of cases. Each episode of infection is commonly followed by breakdown of the skin immune barriers and leads to advanced stages of deep lymphoedema. Systemic septic infections require hospitalization and intensive antibiotic therapy [2]. Lymphatic Filariasis is the second leading cause of disability worldwide. It is one of the debilitating and disfiguring scourges among all diseases. Nearly 120 million people globally are the victims of this scourge. Recurrent attacks of filariasis worsen lymphoedema [3]. Advanced lymphoedema leads to grotesque deformity. Massive chronic manifestations are unfortunately irreversible and lymphatic microsurgery continues to remain an unfulfilled promise.
Chronic venous insufficiency refers to venous dilation and venous reflux of long duration. The clinical signs include minimal superficial dilation to chronic changes with ulceration. Inadequate muscle pump function, incompetent venous valves (reflux) and venous thrombosis or obstruction leads to skin changes, or skin ulceration. Chronic vein abnormalities are present in up to 50 percent of individuals [4].
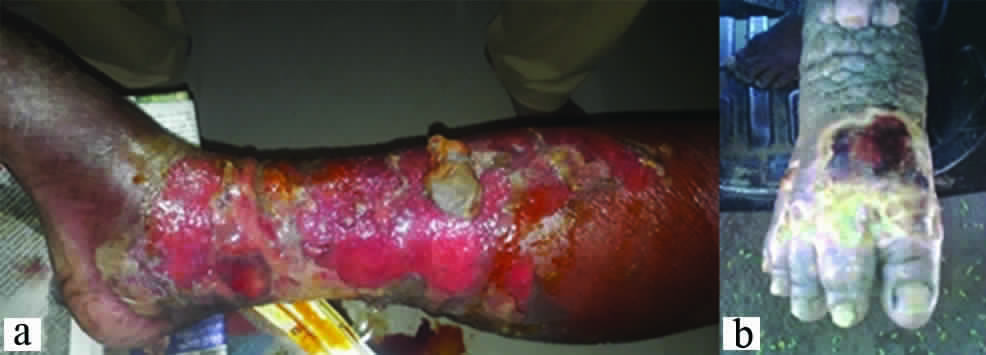
Adeno-Dermato-Lymphangitis (ADL) resembles chronic venous insufficiency. Both the conditions must be diagnosed properly in a filaria endemic zone, because of co-incidence. The two conditions are also common among the diabetic and hypertensive patients. Lymphatic system travels parallel to the cardiovascular system. It contributes to immunological defense when it is functioning correctly. Lymphoedema of leg is caused by insufficient transport of tissue fluid via the lymphatics. This is caused by obliteration of lymphatics after infection, inflammation, subsequent scarring, destruction of valves, and degeneration of muscle cells and obstruction of lumen. There is accumulation of fluid, water, various proteins, migratory immune cells as well as cellular debris in the interstitial space of the subcutaneous tissue between the collagen bundles and around small veins [5]. Presence of lymphoedema increases the risk of infection in the affected area [Table/Fig-3a,b]. It is a chronic condition that mandates lifelong treatment.
Images showing ulceration in a setting of filarial adeno dermatolymphangitis and chronic lymphoedema over: (a) lower limb; and (b) dorsum of foot.
Filariasis is the most common form of secondary lymphoedema worldwide. The parasite Wuchereria bancrofti occupies the lymphatic system and blocks the flow of lymph from the extremity. Stemmer sign is a fairly sensitive and specific sign for lymphoedema. It is positive if the examiner is unable to pinch the skin on the dorsum of the foot [6]. Common laboratory tests are used in diagnosis of ADL in filariasis. OG4C3 test is positive in Bancroftian Filariasis. It detects adult filarial antigen in the blood. It is a quantitative assessment of monoclonal circulating antigen (ELISA) in human lymphatic filariasis. It is highly sensitive and specific in the diagnosis of the disease [7,8]. Antistreptolysin O (ASO Titer) which has high sensitivity is the antibody produced in haemolytic streptococcal infection i.e., group A Streptococcus (GAS) which is commonly associated with ADL [9]. C-Reactive Protein (CRP) is the most widely used indicator of the acute phase protein to detect illness associated with acute and chronic infection. It is more sensitive showing an earlier and intense increase in acute inflammatory process. It disappears with recovery [10].
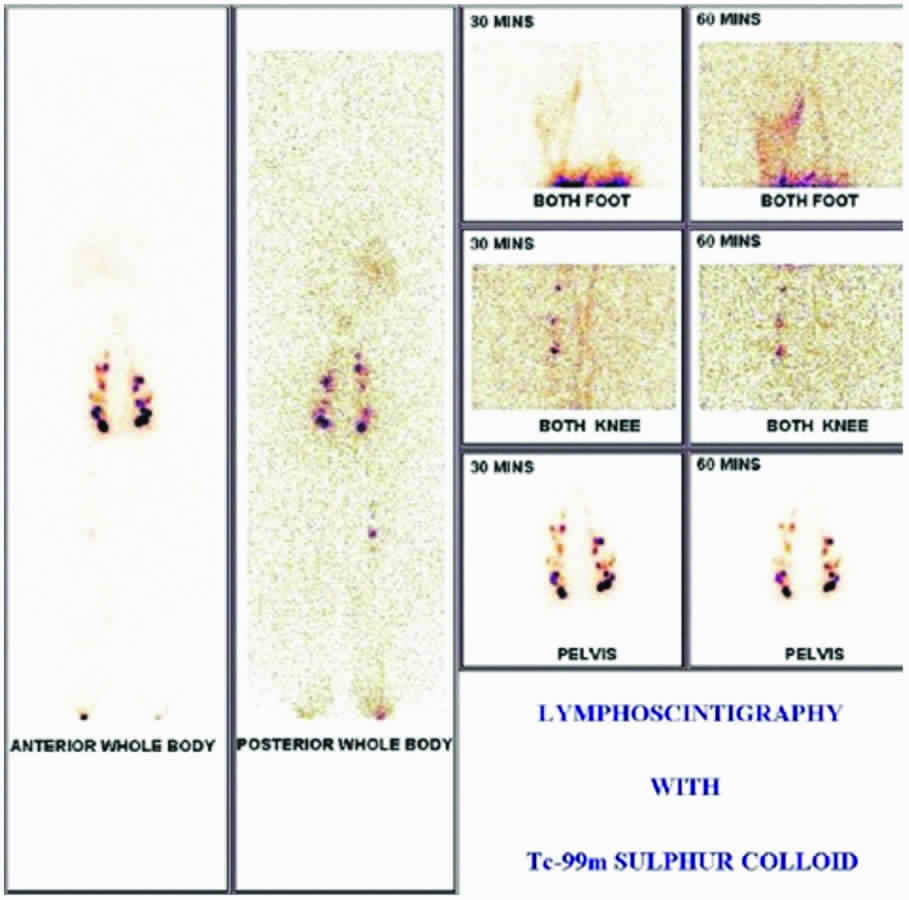
Lymphoscintigraphy is a safe and non-invasive technique to visualize structural and functional changes in lymphatic flow after injection of radiotracer.
It is performed after intra-dermal injection of 2 mCi of Tc-99m-S-colloid in the interdigital space of both feet. Images of the feet, knee and inguinal-para-aortic region are taken after 30 minutes and 60 minutes. This is followed by a whole body scan.
The images of a patient with bilateral ADL after 30 minutes show more lymphatic channels in the right lower leg, which could be due to development of collateral channels around the obstruction. Popliteal lymph nodes are visualized on the right side. There is evidence of partial obstruction of lymphatic flow with reduced visualization of iliac and para aortic lymph nodes. The left side shows partially obstructed lymphatic flow with reduced visualization of iliac and para aortic lymph node channels. Bilateral inguinal lymph nodes are well visualized. The images at 60 minutes do not show any significant increase in tracer uptake in iliac and inguinal lymph nodes along with non-visualization of para aortic lymph nodes on both sides. The liver also shows reduced tracer activity at 60 minutes. Based on these images, findings are suggestive of lymphatic obstruction in both limbs (right>left). The site of obstruction may be in the para aortic region [Table/Fig-4].
Lymphoscintigraphic images in a patient with bilateral adenodermato lymphangitis.
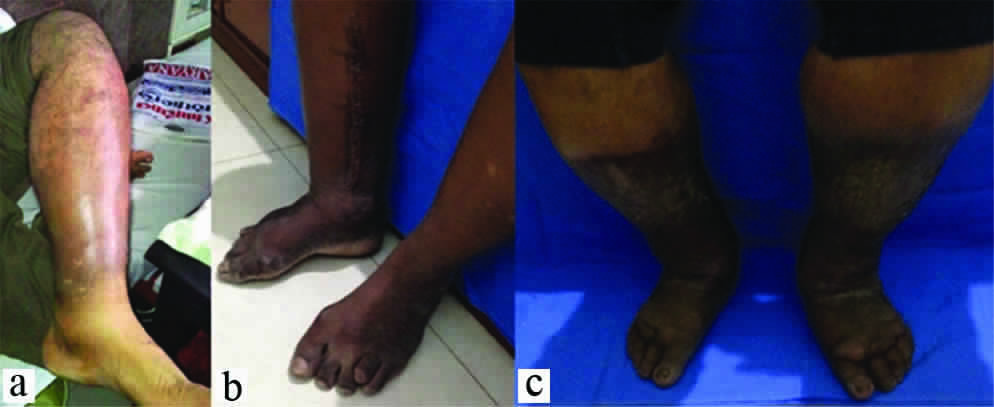
Chronic venous insufficiency refers to the presence of oedema [Table/Fig-5a], skin changes or ulceration [Table/Fig-5b,c] due to venous dilation and venous reflux of long duration. The clinical signs include minimal superficial dilation to chronic changes with ulceration. Inadequate muscle pump function, incompetent venous valves (reflux) and venous thrombosis or obstruction leads to skin changes, or even ulceration. Chronic vein abnormalities are present in up to 50 percent of individuals.
Various grades of chronic venous insufficiency. (a) Note the relative sparing of dorsum of foot in early stages. (b&c) Late stages resemble chronic lymphedema.
Telangiectasia and reticular veins which are dilated intradermal and subdermal veins are the most prevalent abnormalities in 50 to 66 percent individuals with chronic venous insufficiency. Ankle Brachial Pressure Index (ABPI) measurement is needed to exclude peripheral arterial disease in patients with venous insufficiency and ulceration as concurrent arterial disease may be present in both situations [11]. The ABPI is the ratio of the highest ankle to brachial artery pressure. An ABPI between 0.9 and 1.2 is considered normal (free from significant PAD), while a lesser than 0.9 indicates arterial disease [12]. It is performed in patients with weak or absent pulses. In this case compression therapy, which is the standard treatment for venous ulceration, is contraindicated. Duplex ultrasonography can confirm the presence of venous obstruction. It is accurate, reproducible and noninvasive [3]. Photoplethysmography can measure the degree of venous reflux and the efficiency of the calf muscle pump at rest and after exercise [10].
ADL is the most common precursor of foot ulcer in secondary lymphoedema due to filariasis. Chronic venous insufficiency associated with oedema, pigmentation and ulcer of the foot resembles foot ulcer following ADL. The clinical diagnosis can be made on the basis of the following features [Table/Fig-6].
Table showing comparison between Dermato-Lymphangitis and Infected venous insufficiency.
| S. No. | Clinical Findings and Tests | Dermato Lymphangitis | Infected Venous Insufficiency |
|---|
| 1 | Pain, oedema, Non-healing wounds | May be present | Usually present |
| 2 | Pigmentation of skin | Uncommon | Common |
| 3 | Stemmer Sign | Positive | Negative |
| 4 | Lymphoscintigraphy | Suggestive | Non-Suggestive |
| 5 | Og4C3 Test | Positive | Negative |
| 6 | Duplex Ultrasonography | Negative | Confirmatory |
| 7 | D.V.T., Varicose vein | Absent | May be present |
| 8 | Diabetes & Hypertension | May be associated | Usually present |
| 9 | Telangiectasias | Absent | Present |
| 10 | Ankle-Brachial Index | Negative | Positive |
Conservative treatment for lymphoedema is successful in most cases. Each episode of dermato-lymphangitis is commonly followed by worsening of limb swelling. The recurrence of acute attacks is higher in subjects with long duration of oedema. The lymphatic system needs pressure to help it work, but too much pressure can be damaging. Once lymphatic system has been compromised, there is no way to regrow or repair the same. The infection can spread quickly in lymphoedema. So, it is vital to start antibiotic therapy immediately. The antibiotic of choice is a combination of amoxicillin and clavulanic acid in cases with mild infection. Complicated infections can be treated with fourth generation cephalosporins or quinolones. Fungal infection subsequent to bacterial infection should be managed with topical antifungal medication such as Mycostatin. Surgical therapy for lymphoedema is largely disappointing. Outcome after operation in the lower extremity is significantly less favourable [13].
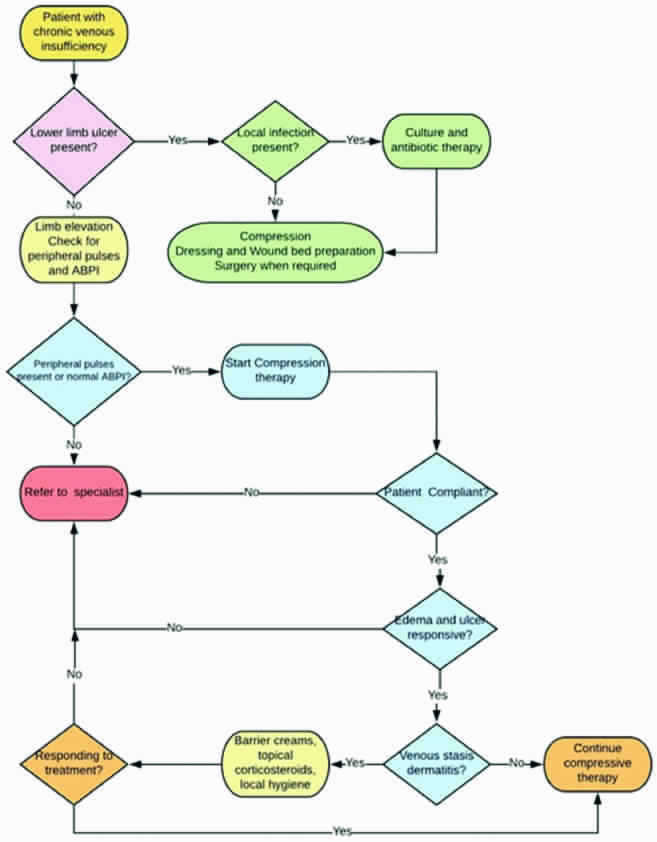
The treatment of chronic venous insufficiency [Table/Fig-7] is directed towards the correction of venous hypertension, relief of pain, reduction in oedema, control of infection, improvement of lipoderma to sclerosis, healing of ulceration, and prevention of recurrence. Compression therapy improves venous hypertension, thereby reducing oedema and ulceration. Ulcer healing by systemic antibiotics is not efficacious and can be used when complicated with cellulitis. These wound are not typically infected. Topical antiseptic such as povidone-iodine are tissue toxic and will retard wound healing. Pentoxifylline may be effective for treatment of venous ulceration by mediating cytokine production, which results in antithrombotic effects. Flavonoids (100 mg daily) decreases white cell adhesion and increases red cell velocity. Aspirin (enteric 300 mg daily) has been associated with increase rate of wound healing. Surgical treatment of the ulcer is recommended with skin graft and venous incompetence by micro-surgical transfer of well-vascularized tissue.
Algorithm for approach to chronic venous insufficiency.
Conclusion
Chronic venous insufficiency appears similar to lymphatic filariasis of the lower extremity. Regular foot hygiene, appropriate antibiotic therapy and elastic compression stocking will help the patients lead a normal life in patients with lymphoedema. Lymphoedema does not cause chronic skin ulcers. If ulceration occurs; it is due to concomitant venous insufficiency. Although the clinical presentations among the two are indistinguishable, an attention to details and appropriate investigations would guide the treating physician in the management plan.
[1]. Damstra RJ, Van S MA, Boomsma JH, Nelemans P, Veraart JC, Erysipelas as a sign of subclinical primary lymphooedema: a prospective quantitative scintigraphic study of 40 patients with unilateral erysipelas of the legBr J Dermatol 2008 158(6):1210-15.10.1111/j.1365-2133.2008.08503.x18363756 [Google Scholar] [CrossRef] [PubMed]
[2]. Dreyer G, Addiss D, Gadelha P, Lapa E, Williamson J, Dreyer A, Interdigital skin lesions of the lower limbs among patients with lymphoedema in an area endemic for bancroftian filariasisTrop Med Int Health 2006 11(9):1475-81.10.1111/j.1365-3156.2006.01687.x16930270 [Google Scholar] [CrossRef] [PubMed]
[3]. Panda DK, Mohapatra DP, Mohapatra MM, Breast filariasis or inflammatory breast carcinoma? Reaching a diagnosisBMJ Case Rep 2015 2015pii: bcr2015212254. doi: 10.1136/bcr-2015-21225410.1136/bcr-2015-21225426567240 [Google Scholar] [CrossRef] [PubMed]
[4]. Barwell JR, Davies CE, Deacon J, Harvey K, Minor J, Sassano A, Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study): randomised controlled trialLancet 2004 363(9424):1854-59.10.1016/S0140-6736(04)16353-8 [Google Scholar] [CrossRef]
[5]. Olszewski WL, Jain P, Zaleska M, Stelmach E, Swoboda E, Chronic lower limb wounds evoke systemic response of the lymphatic (immune) systemIndian J Plast Surg 2012 45(2):255-60.doi: 10.4103/0970-0358.10128910.4103/0970-0358.10128923162224 [Google Scholar] [CrossRef] [PubMed]
[6]. Greene, Arin K, Slavin, Sumner A., Brorson, Håkan (Eds.), Lymphedema: Presentation, Diagnosis and Treatment, Springer 2015, 122 Pp. Available from https://www.springer.com/in/book/978331914492410.1007/978-3-319-14493-1 [Google Scholar] [CrossRef]
[7]. Panda DK, Mohapatra DP, Mohapatra MM, Filarial breast lumpBMJ Case Rep 2017 doi:10.1136/bcr-2017-22153610.1136/bcr-2017-22153628835430 [Google Scholar] [CrossRef] [PubMed]
[8]. Lalitha P, Ravichandran M, Suba S, Kaliraj P, Narayanan RB, Jayaraman K, Quantitative assessment of circulating antigens in human lymphatic filariasis: a field evaluation of monoclonal antibody-based ELISA using blood collected on filter stripsTrop Med Int Health 1998 3(1):41-45.10.1046/j.1365-3156.1998.00170.x9484967 [Google Scholar] [CrossRef] [PubMed]
[9]. Rantz LA, Randall E, Rantz HH, Antistreptolysin “O”, a study of this antibody in health and in hemolytic streptococcus respiratory disease in manAm J Med 1984 5:3-23.10.1016/0002-9343(48)90003-5 [Google Scholar] [CrossRef]
[10]. Sproston NR, Ashworth JJ, Role of C-reactive protein at sites of inflammation and infectionFrontiers in Immunology 2018 9:75410.3389/fimmu.2018.0075429706967 [Google Scholar] [CrossRef] [PubMed]
[11]. Alquire PC, Mathes BM, Medical management of lower extremity chronic venous diseaseaccessed from https://www.uptodate.com/contents/medical-management-of-lower-extremity-chronic-venous-disease/print on 20th may 2018 [Google Scholar]
[12]. Vowden P, Vowden K. Doppler assessment and ABPI interpretation in the management of leg ulceration. Accessed from http://www.worldwidewounds.com/2001/march/Vowden/Doppler-assessment-and-ABPI.html on 24 may 2018 [Google Scholar]
[13]. Campisi C, Boccardo F, Tacchella M, Reconstructive microsurgery of lymph vessels: the personal method of Lymphatic-Venous-Lymphatic (LVL) interpositioned grafted shuntMicrosurgery 1995 16(3):161-66.10.1002/micr.19201603097637625 [Google Scholar] [CrossRef] [PubMed]