Rheumatoid arthritis is a chronic systemic autoimmune disease of unknown aetiology with the inflammatory defect of synovial tissue, development of fibrinous tissue (pannus), destruction of synovial structure (destruction of cartilage and underlying bone) and periarticular tissue [1]. RA is an actual medical and socio-economical problem as it is widespread and a leading cause of temporary or permanent loss of ability to work when concerned with the majority of the working population.
The basic inflammatory mechanism in RA is the migration of leukocytes, neoangiogenesis and tissue remodeling, which is implemented by the participation of adhesion molecules expressed by the endotheliocytes and immunocompetent cells, and results in their interaction as a response to inflammatory stimulus. By acting as receptors, adhesins increase immunological response by stimulating intercellular interactions and adhesion of cells to matrix proteins in the intercellular space [2,3].
Selectins, integrins, cadherins and immunoglobulin superfamily comes under adhesion molecules. Selectins and its ligands are involved in the rolling of leukocytes on the endothelial surface, immunoglobulin superfamily in coordination with integrins affect the activation, adhesion of cells to endothelium and their penetration through subendothelial space to the focus of inflammation [2,4].
Migration of leukocytes is impossible without the involvement of ICAM-1, which facilitates transendothelial migration of cells to the focus of inflammation and interaction of antigen presenting cells with T-lymphocytes [3,5]. Hyperexpression of ICAM-1 induced by proinflammatory cytokines (tumour necrosis factor-α, interleukin-1, interferon-γ) on endotheliocytes and fibroblasts are responsible for the initiation and synchronisation of the inflammatory reaction in joint tissue in RA [6].
There is no doubt about the increased blood concentrations of ICAM-1 in patients with RA [7,8]. Likely, increased serum concentrations of ICAM-1, Vascular Cell Adhesion Molecule-1 (VCAM-1) and vascular endothelial growth factor in RA depend on the histological manifestation of synovitis and were correlated with levels of ESR and C-reactive protein [9]. At the same time, a possible interrelation of ICAM-1 with duration of disease, activity of the process, immunological alterations, systemic effects and complications of RA was debated [7,8,10]. It was assumed that on the basis of revealed contradictions genetic polymorphism of ICAM-1 might be associated with the pathology of joints [8]. The present study aimed to study the dynamics of concentrations of ICAM-1 in blood depending on the course of RA, evaluating the role of ICAM-1 in the pathological mechanism of development of RA and in the progression of RA.
Materials and Methods
The present research was of observational, case-control type, followed the STROBE guidelines, and was conducted from September 2015 to June 2017. A total of 134 patients with RA (30 male and 104 female) of age 20 to 66 years (mean age 50.08±0.97) were examined, who were under observation and undergoing treatment at Department of Rheumatology and rheumatologist cabinet of Stavropol regional clinical hospital, Stavropol state, Russia. The research corresponded with the Helsinki declaration of world medical association on Ethical Principles for medical research involving human subjects. All the participants provided informative consent for conducting the research. The research was approved by Ethical Committee of Stavropol state medical university, Stavropol, Russia.
Control group constituted of 70 practically healthy people, comparable to sex, age, physical development and concomitant pathologies with cases, who were undergoing general health checkup at the clinic of Stavropol state medical university, Stavropol, Russia. The Sample size was calculated with the confidence level of 95% and with the confidence interval of 8.13.
Inclusion Criteria: Patients with RA of age 18 years and elder, consent to participation in the research, intake of NSAIDs and glucocorticoids in stable doses for not less than four weeks.
Exclusion criteria: Joint disease of other aetiology, intake of genetically engineered biological drugs or other disease modifying drugs, acute and chronic somatic diseases in the period of exacerbation, infections, benign neoplasms, refusal to participate in the research.
Measurements: Diagnosis of RA was made in accordance with the classification criteria of ACR/EULAR (2010) [11]. Clinical characteristics of the patients in accordance with the classification adopted by the association of rheumatologists of Russia is presented in [Table/Fig-1].
Clinical characteristics of patients with rheumatoid arthritis.
| Indicator | No. of patients, n(%) |
|---|
| Age (years) |
| <45 years | 37 (27.6%) |
| ≥45 years | 97 (72.4%) |
| Sex |
| Male | 30 (22.4%) |
| Female | 104 (77.6%) |
| Clinical stage |
| Early | 3 (2.2%) |
| Extended | 22 (16.4%) |
| Late | 109 (81.4%) |
| Immunological characteristics |
| Seropositive (RF present) | 120 (89.6%) |
| Seronegative (RF absent) | 14 (10.4%) |
| ACCP-positive | 93 (76.8%) |
| ACCP-negative | 28 (23.2%) |
| Stage of activity (by index DAS 28) |
| Moderate | 44 (32.8%) |
| Severe | 90 (67.2%) |
| X-ray stage |
| Stage I | 7 (5.2%) |
| Stage II | 18 (13.4%) |
| Stage III | 95 (70.9%) |
| Stage IV | 14 (10.5%) |
| Presence of erosions on bone |
| Erosive RA | 117 (87.3%) |
| Non-erosive RA | 17 (12.7%) |
| Functional class |
| I | 4 (3.0%) |
| II | 66 (49.2%) |
| III | 64 (47.8%) |
| Systemic manifestations |
| Present | 23 (17.2%) |
| Absent | 111 (82.8%) |
| Complications |
| Present | 89 (66.4%) |
| Absent | 45 (33.6%) |
| Haematological abnormalities |
| Anemia | 63 (47.0%) |
| Thrombocytosis | 19 (14.2%) |
| Osteocalcin |
| Normal | 58 (75.3%) |
| Decreased | 19 (24.7%) |
| Score |
| <5 | 87 (77.7%) |
| ≥5 | 25 (22.3%) |
All the patients had undergone a complex clinico-functional, laboratory, instrumental and immunological examinations. Plasma concentrations of ICAM-1 were analysed by ELISA test using kits of “Bender Med Systems GmbH” (Austria) in accordance with the provided instructions.
Statistical Analysis
Statistical analysis of the obtained results was performed using program pack, adapted for medico-biological researches and with program IBM SPSS statistics 24.0. Two sample Student’s t-criterion and criteria of Newman-Keuls were evaluated and correlation analysis with application of Pearson (r) and Spearman (rs) criteria were used. Results were considered reliable with the level of significant difference p≤0.05.
Results
It can be seen from the [Table/Fig-1] that in the research women more than 45 years of age prevailed, having the late stage of the disease, high level of activity and are characterised with the presence of Rheumatoid Factor (RF), Antibody to Cyclic Citrullinated Peptide (ACCP) in blood. Duration of joint syndrome constituted 11.06±0.72 years. Mean concentrations of RF IgM and ACCP were 173.67±21.79 ME/mL and 332.53±38.88 IU/mL respectively. Most of the patients were diagnosed with an erosive form of the disease (87.3%), x-ray stage III (70.9%) and II and III functional class (49.2% and 47.8% respectively). In 17.2% of the patients, systemic manifestations (mostly rheumatoid nodules) of the disease and in 2/3 of the cases complications of the disease (secondary osteoarthrosis) were observed. Along with the above analyses, levels of serum osteocalcin, as a marker of bone metabolism, were analysed in 77 patients. Serum concentration of osteocalcin less than the lower limits of the norm was seen in 23% of patients (p<0.05) and mean levels were 9.63±0.59 ng/mL.
At the time of inclusion in the research, the total cardiovascular risk with the help of SCORE chart modified by EULAR in 112 patients who were above 40 years of age was studied [12]. Mean values of index SCORE were 2.69±0.31 (moderate risk), and in 22.3% of the patients were evaluated as having high and very high cardiovascular risk.
In RA significant increase in the concentrations of ICAM-1 in the blood (p<0.05) was observed. The increased, plasma concentration of the mediator of intercellular adhesion was not associated with age and gender of the patients. Also, significant dependence of ICAM-1 levels with presence or absence of RF and ACCP was not detected.
In patients with a late clinical stage of the disease values of adhesion molecules were higher (p<0.05), than in the early stage of RA. A positive correlation of ICAM-1 with the clinical stage of the disease (rs=+0.36, p<0.05) was observed [Table/Fig-2]. The severe activity of the disease, determined by index DAS 28 was characterised by very high levels of adhesins in the blood (p<0.05), than that of with minimum or moderate level of activity. Levels of ICAM-1 were positively correlated with the stage of activity of RA, values of ESR and C-reactive protein (rs=+0.27; rs=+0.17; rs=+0.24, p<0.05 respectively) [Table/Fig-3].
Concentration of ICAM-1 in patients with RA (values mentioned are in mean and standard deviation).
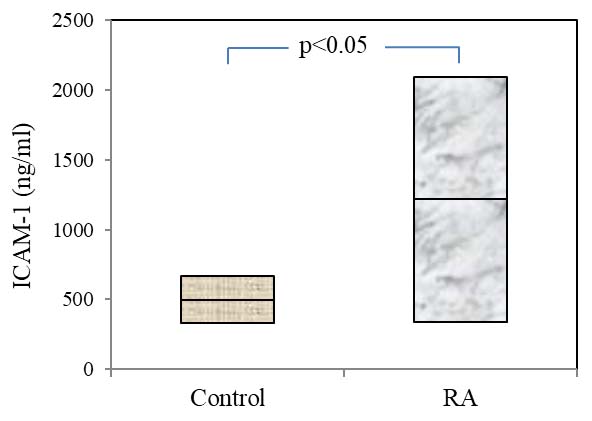
Mean values of ICAM-1 in control group and in patients with rheumatoid arthritis.
| Indicator | ICAM-1 (ng/mL) |
|---|
| Control | 499.04±20.24 |
| Rheumatoid arthritis | 1316.73±94.19 * |
| RA clinical stage |
| Early and Extended | 895.36±139.04 * |
| Late | 1291.97±86.15 */† |
| Immunological characteristics |
| Seropositive (RF present) | 1208.03±80.61 * 1 |
| Seronegative (RF absent) | 303.29±22.34 * |
| Immunological characteristics |
| ACCP-positive | 1248.08±86.67 * |
| ACCP-negative | 1155.07±163.93 * |
| Stage of activity (by index DAS 28) |
| Moderate | 1037.39±112.24 * |
| Severe | 1306.27±97.53 */† |
| X-ray stage |
| Stage I and II | 998.44±148.94 * |
| Stage III and IV | 1268.33±86.15 * |
| Presence of erosions on bone |
| Erosive RA | 1258.54±82.63 * |
| Non-erosive RA | 938.82±171.11 * |
| Functional class |
| I and II | 1110.34±95.70 * |
| III | 1335.70±118.09 * |
| Systemic manifestations |
| Present | 1497.22±212.77 */† |
| Absent | 1160.12±79.46 * |
| Complications |
| Present | 1173.31±89.24 * |
| Absent | 1306.30±140.81 * |
| Osteocalcin |
| Normal | 1229.66±101.03 * |
| Decreased | 1582.53±218.91 */† |
| Score |
| <5 | 1126.89±85.83 * |
| ≥5 | 1524.96±219.00 */† |
* p<0.05 in comparison with control, † - p<0.05 in comparison within the groups
Indicators of mediators of intercellular interaction were independent of severity of x-ray changes of joints, including presence or absence of erosions, and also were not associated with functional class of RA. Nevertheless, concentrations of ICAM-1 positively correlated with x-ray stage and functional class of RA (rs=+0.32; rs=+0.19; p<0.05 respectively).
In RA, levels of adhesion molecule were increased irrelevant of disorder of bone metabolism. In cases with decreased synthesis of osteocalcin, blood concentrations of ICAM-1 were significantly higher (p<0.05), than in cases with a normal level of osteocalcin in blood.
In patients with RA, values of modified EULAR SCORE scale ≥5 points, concentrations of ICAM-1 were significantly higher (p<0.05) than in the patient group with low and moderate 10-year cardiovascular mortality risk.
Discussion
The observed increase of ICAM-1 in patients’ blood with RA coincides with the earlier received data [7-9]. At the same time, absence of change in serum concentrations of adhesins in cases with uncomplicated RA was published by many authors. Even though significant dependence of ICAM-1 with RF and ACCP was not detected in the present study, there is a point of view in some researches that high levels of adhesion molecules are observed in cases with ACCP positive variant of the disease [7,10]. The positive correlation of ICAM-1 with the clinical stage of RA specifies its significance in the inflammatory process and progression of the disease. However, according to some research, it was observed that in cases with a duration of the disease more than 10 years, the concentration of ICAM-1 in blood reciprocally decreases while compared to the cases of RA with duration of disease less than 2 years [7].
The observed correlation of ICAM-1 with acute phase inflammatory proteins not only indicates its relation with severity of pathological process but also confirms the significance of the mediator in mechanism of inflammation in RA [9,13].
Presence of systemic manifestations of disease led to a significant increase in levels of ICAM-1 in the blood (p<0.05). Although, there is an opinion, that indicators of soluble ICAM-1 were not related to extra articular manifestations [14] and even showed decrease in patients with symptoms of cutaneous vasculitis [8].
Disorder of bone tissue metabolism in the form of decreased levels of osteocalcin in blood, associated with increased levels of adhesins may be due to their involvement in the regulation and arrest of osteoblastic cell cycles in cases with chronic inflammation as seen in RA [15].
Correlation of adhesion molecules with visceral manifestations in RA may be due to de novo expression of VCAM-1 and intensification of expression of ICAM-1 on the vascular endothelium under the influence of increased levels of proinflammatory cytokines (tumour necrosis factor-α), which are determined in the present group of patients [13,16].
In patients with thrombocytosis, plasma concentration of ICAM-1 were higher (p<0.05) than in the patients with normal levels of thrombocytes in blood, suggesting the complicity of adhesion molecules with haematological abnormalities in joint pathologies.
In several researches, it was observed that there was an increase in the production of ICAM-1 and decrease in endothelial-dependent and endothelial independent vasodilation with the intensification of activity of RA [7,10]. Correlation of mediators of intercellular interactions with very active and aggressive course of RA not only approves the role of endothelial dysfunction and chronic systemic inflammation in its progression and might be the basis of development of cardiovascular pathologies considering the common links of pathogenesis [17-19]. So, in RA risk of development of cardiovascular diseases was increased by 50%, and mortality increased twice than that in the general population [18,19]. Paying attention to this fact, adhesion molecules may not only be associated with changes in synovial tissue, but also with the progression of atherosclerosis on vascular walls. Therefore, patients with high activity of RA and systemic manifestations of RA must be isolated under the risk group of the development of cardiovascular events, and further researches are necessary to evaluate the predictive ability of adhesion molecules on the disease activity and course of disease by evaluating the levels of ICAM-1, not only at the moment of diagnosing the disease but also during the course of treatment with DMARDs and biological agents.
Conclusion
In RA, increased levels of ICAM-1 in blood were observed regardless of sex and age of the patients. Maximum rise in the level of adhesion molecules was observed in cases with high activity of the disease, with systemic manifestations and haematological abnormalities, which indicates the pathogenic relevance of the mediator in initiation and progression of the disease. Disorder of bone tissue metabolism in the form of decreased osteocalcin levels suggests involvement of adhesins in maintaining bone homeostasis and its remodeling. In RA, the interrelation of circulating ICAM-1 with values of SCORE scale characterises the role of endothelial adhesive function in the development of cardiovascular diseases.
* p<0.05 in comparison with control, † - p<0.05 in comparison within the groups