Malaria is a serious health problem in sub-tropical and tropical regions of the world. India accounts for the third highest number of cases in the world and about 70% of all malaria cases in South-East Asian region [1]. Mangalore city situated in southern part of India is highly endemic for malaria. The annual incidence of malaria over here, for the year 2016, was reported to be 11037 cases of which 9696 cases were due to Plasmodium vivax. This accounted for almost 70% of the cases from the entire state of Karnataka [2]. In such highly endemic areas, rapid and efficient diagnostic methods are needed for early diagnosis and management of malaria.
Non-testing before treatment often results in extensive overuse of anti-malarial drugs [3,4], which has economic implications, drug resistance [4,5] and may even border on safety as far as the individual is concerned. Therefore since 2010, the World Health Organisation (WHO) recommended that anti-malarial treatment can be initiated only after parasitological confirmation of suspected cases [5].
Malaria Rapid Diagnostic Test (MRDT) has several advantages for screening activities. It is easily accessible, cost-effective, easy to perform and interpret, easy to transport and provides results immediately [6,7]. It is thus suitable for usage in malaria-endemic areas, settings with limited health personnel and facilities, and during outbreaks [8]. Its usage has been reported to significantly reduce referrals and inpatient’s length of hospital stay [9].
On the other hand microscopy requires electricity, well-trained technicians and rigorous maintenance of functional infrastructures [10,11].
However, concern about the accuracy of MRDTs has made its wide-scale usage a debatable issue [12,13]. Discrepancies have been observed in MRDT sensitivities in several observational studies [14-16]. The validity of RDT kits is commonly measured by its sensitivity and specificity. It is a very critical situation wherein anti-malarial drugs are denied to patients with malaria due to False Negative (FN) test results. On the other hand, drugs are dispensed unnecessarily for treating patients due to False Positive (FP) test results [17]. The present study was therefore aimed at assessing the validity of antigen detecting MRDTs by comparing the test results with that of conventional Giemsa stained thick and thin blood film microscopy.
Materials and Methods
The present cross-sectional study was conducted from May 2017 to September 2017 among 309 febrile patients who were directed to the laboratory of Malaria Control Cell of Municipal Corporation and Government Wenlock Hospital at Mangalore, Karnataka state in southern India. The study period corresponds to the peak season of the outbreak of malaria in Mangalore.
Institutional Ethics Committee Approval was taken for the conduct of the study. The approval number being IEC KMC MLR 05-17/98. Permission to conduct the study at the above mentioned facilities were taken from Commissioner of Municipal Corporation and District Surgeon of Government Wenlock Hospital, Mangalore respectively.
Sample size of minimum 200 participants was calculated based on the reported sensitivity of 79% of Rapid Diagnostic Test (RDT) for diagnosing malaria as reported in a previous study [18] and prevalence of malaria cases in Mangalore taken as 1.52% [2].
The nature and purpose of the study were explained to participants in the language they can understand. Written informed consent was obtained from patients aged ≥18 years and those who were willing to participate in the study. For minors, assent for their participation was taken by obtaining consent from their guardians accompanying them. Participants were enrolled in the present study using purposive sampling method.
All patients screened at these laboratories during the study period, and also those who gave consent for participation were included in this study. Seriously ill patients and those refusing or unable to provide informed consent were excluded from the study.
Each case of fever underwent both MRDT and blood smear examination. Microscopic examination of stained blood smears was taken as “gold standard” for detection of malaria parasitaemia. This method has been reported to detect a parasitaemia as low as 0.0001% [19].
To perform these tests the left index finger was first cleaned using a non-alcohol swab. A blood specimen was then collected from finger-prick using a sterile lancet by a laboratory technician. One drop of blood was collected in a capillary tube and transferred into a well on the RDT kit. Then three drops of assay buffer were added into the assay buffer well. The result was read after 20 minutes [10].
The MRDT kit used in the present study was the SD Bioline Malaria Antigen test kit (Standard Diagnostics Inc., India). It has a control line and also a test line which targets either histidine-rich protein II or lactate dehydrogenase of Plasmodium [20].
An additional 2-3 drops of blood were collected from the same finger pick for preparation of a thick blood smear and 2-4 drops for thin smear. Both thick and thin blood smears were prepared on the same slide. The films were properly dried and stained with 10% Giemsa solution, then washed after 10 minutes using distilled water. The films were then dried in a vertical position. A drop of immersion oil was applied on the dried stained slide and examined microscopically for malaria parasites using a 100X objective lens. Smears were considered negative when no parasites were detected after examination of 100X high power microscopic fields [21,22]. When Plasmodium was identified in the thick smears, the species were differentiated from the thin smears.
Statistical Analysis
Data were entered and analysed using Statistical Package for Social Sciences (SPSS) Inc., Chicago, IL, USA Version 11.0. Proportions were calculated and the diagnostic performance was determined by calculating the test sensitivity, specificity, predictive values, accuracy rate, False Positive Rate (FPR), False Negative Rate (FNR), Diagnostic Likelihood Ratio (DLR), Diagnostic Odds Ratio (DOR), False Omission Rate (FOR), False Discovery Rate (FDR) and Receiver Operating Characteristics (ROC) curve. Statistical test like Chi-square test was used to test association. A p-value less than 0.05 was considered as statistically significant association.
Results
A total of 210 cases were diagnosed with malaria by Peripheral Smear (PS) examination. This comprised of 181 cases of vivax malaria, 21 cases of falciparum malaria and 8 cases of mixed infection. Therefore, the total proportion of cases with Plasmodium vivax malaria was 189 (90%).
Sensitivity calculated as True Positive (TP)/(TP+FN)×100 was 98.6%, specificity calculated as True Negative (TN)/(TN+FP)×100 was 86.9%, Positive Predictive Value (PPV) calculated as TP/(TP+FP)×100 was 94.1%, Negative Predictive Value (NPV) calculated as TN/(TN+FN)×100 was 96.6% [Table/Fig-1].
Validity of rapid diagnostic test for detection of all types of malaria.
| Test results | PS* positive | PS negative | Total |
|---|
| RDT** positive | 207 (TP) | 13 (FP) | 220 |
| RDT negative | 3 (FN) | 86 (TN) | 89 |
| Total | 210 | 99 | 309 |
*Peripheral smear, **Rapid diagnostic test
Accuracy rate calculated as (TP+TN)/total no. of tests×100 was 94.8%.
FPR calculated as FP/(FP+TN)×100 was 13.1%. FNR calculated as FN/(FN+TP)×100 was 1.4%.
Positive likelihood ratio of the test calculated as sensitivity/(1-specificity) was 7.53. The negative likelihood ratio of the test calculated as (1-sensitivity)/specificity was 0.016. DOR which is calculated as the ratio of positive upon negative likelihood ratio was 470.6.
The False Discovery Rate (FDR) calculated as (1-PPV)X100 was 5.9%. The False Omission Rate (FOR) calculated as (1-NPV)X100 was 3.4% [Table/Fig-1].
Sensitivity and specificity of RDT were found to be 98.5% and 85.6% respectively among adults. Among other patients aged below 18 years, these parameters were found to be 100% [Table/Fig-2].
Comparison of validity of rapid diagnostic test for detection of all types of malaria among different age groups of participants (n=309).
| Adults (age ≥18 years) | PS positive | PS negative | Total |
|---|
| RDT positive | 195 (TP) | 13 (FP) | 208 |
| RDT negative | 3 (FN) | 77 (TN) | 80 |
| Total | 198 | 90 | 288 |
| Age<18 years |
| RDT positive | 12 (TP) | 0 (FP) | 12 |
| RDT negative | 0 (FN) | 9 (TN) | 9 |
| Total | 12 | 9 | 21 |
For diagnosis of Plasmodium vivax, sensitivity of MRDT was 98.9%, specificity was 86.9%, PPV was 93.2% and NPV was 97.7% [Table/Fig-3].
Validity of rapid diagnostic test for detection of Plasmodium vivax malaria.
| Test results | PS positive | PS negative | Total |
|---|
| RDT positive | 179 (TP) | 13 (FP) | 192 |
| RDT negative | 2 (FN) | 86 (TN) | 88 |
| Total | 181 | 99 | 280 |
For diagnosis of Plasmodium falciparum, sensitivity of MRDT was 100%, specificity was 86.9%, PPV was 61.8% and NPV was 100% [Table/Fig-4].
Validity of rapid diagnostic test for detection of Plasmodium falciparum malaria.
| Test results | PS positive | PS negative | Total |
|---|
| RDT positive | 21 (TP) | 13 (FP) | 34 |
| RDT negative | 0 (FN) | 86 (TN) | 86 |
| Total | 21 | 99 | 120 |
For diagnosis of mixed malaria cases, sensitivity of MRDT was 87.5%, specificity was 86.9%, PPV was 35% and NPV was 98.9% [Table/Fig-5].
Validity of rapid diagnostic test for detection of mixed malaria.
| Test results | PS positive | PS negative | Total |
|---|
| RDT positive | 7 (TP) | 13 (FP) | 20 |
| RDT negative | 1 (FN) | 86 (TN) | 87 |
| Total | 8 | 99 | 107 |
Out of 210 confirmed cases of malaria, majority 116 (55.2%) were of the age group 16-35 years and 124 (59%) were males. There was no association between age (p=0.435) and gender (p=0.441) of participants with presence of malaria [Table/Fig-6].
Association between age and gender of participants with presence of malaria as diagnosed by peripheral smear examination.
| Socio-demographic variables | Malaria present | Malaria absent | Total |
|---|
| Age group (years) |
| ≤15 | 8 (57.1) | 6 (42.9) | 14 |
| 16-25 | 58 (63.7) | 33 (36.3) | 91 |
| 26-35 | 58 (77.3) | 17 (22.7) | 75 |
| 36-45 | 30 (65.2) | 16 (34.8) | 46 |
| 46-55 | 17 (63) | 10 (37) | 27 |
| 56-65 | 13 (76.5) | 4 (23.5) | 17 |
| 66-75 | 17 (73.9) | 6 (26.1) | 23 |
| >75 | 9 (56.2) | 7 (43.8) | 16 |
| Chi-square and p-value | χ2=6.94, p=0.435 |
| Gender |
| Males | 124 (66.3) | 63 (33.7) | 187 |
| Females | 86 (70.5) | 36 (29.5) | 122 |
| Chi-square and p-value | χ2=0.593, p=0.441 |
| Total | 210 | 99 | 309 |
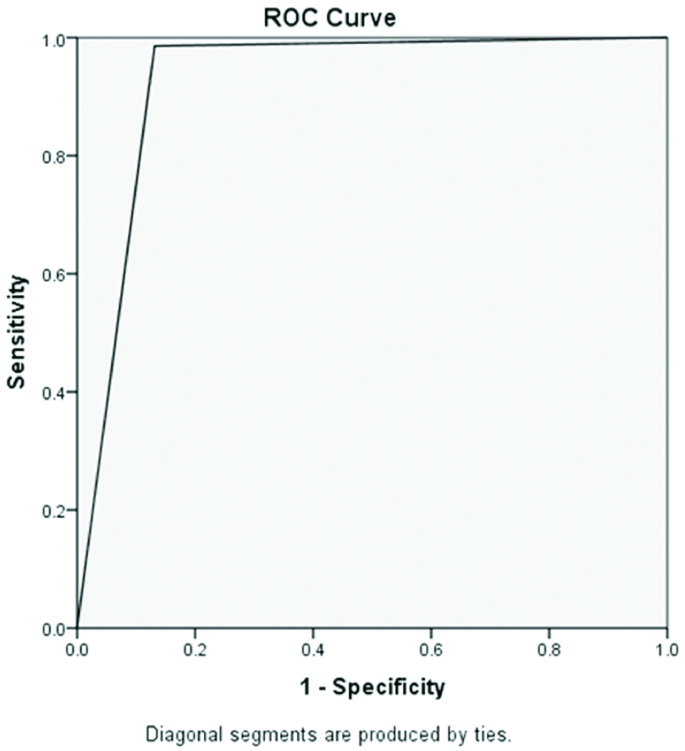
The [Table/Fig-7] shows the Area Under the Curve (AUC) as 0.927, indicating that the agreement between MRDT and PS was seen in 92.7% of cases.
Area under the Receiver Operating Characteristics (ROC) curve for malaria rapid diagnostic test in comparison to the peripheral blood smear examination.
Discussion
More than half of the cases in the present study were of the age group of 16-35 years. In a Nigerian study, it was reported that the infection rate was highest in the age group 0-11 years (81.1%) followed by 26-49 years (69%) [23]. The latter study reported all malaria cases due to Plasmodium falciparum in contrast to present observations where 90% of cases were due to Plasmodium vivax [24].
In previous studies, the sensitivity of RDT for diagnosis of malaria ranged from 37.7% to 98.8% in comparison to 98.6% reported in this study [10,22-26]. The sensitivity reported in this study is above the 95% value recommended by World Health Organization [27]. The high sensitivity of MRDT kit implies that it is less likely to fail in diagnosing a positive patient as having the disease.
In previous studies, specificity of RDT for diagnosis of malaria ranged from 87.1% to 98.7% in comparison to 86.9% reported in this study [10,22-26]. Its relatively comparable specificity in the present study with that of other studies shows that a patient is not incorrectly diagnosed as having a malaria parasite, thus avoiding misdiagnosis of other febrile illnesses.
The present study found FP results in 13 cases. A high number of FPs would reduce the specificity of the screening test. FP may be attributed to residual malarial antigens arising from a previous infection which can test positive with rapid test kit [28,29]. Other factors like high humidity can rapidly degrade nitrocellulose capillary flow action in MRDT kits [19,30]. High temperatures in the tropics would also result in denaturation of antibodies in the test membrane of MRDT kits resulting in impaired binding to the target antigens [31]. Similar to moisture, heat damages the nitrocellulose membrane forming the strip thus, changing its flow characteristics or causes the antibody to detach from the membrane [19,31]. Mangalore has a tropical climate with warm and humid conditions. Therefore, the health care providers in this setting should take extra precautions in the appropriate storage of RDT kits to ensure its quality.
Rare cases of FN identified in the study could be due to low parasite densities in the blood [19].
PPV was reported as 31.5% to 98% in comparison to 94.1% reported in this study [10,22-26]. NPV was reported as 34 to 99.8% in comparison to 96.6% reported in this study [10,22-26]. These again indicate good performance of the MRDTs.
The accuracy rate of test in the study was 94.8% compared to 51% [10], 92.5% [22] and 96% [23] reported in other studies. This also supports good agreement between MRDT and blood smear examination.
FPR and FNR in a study done in Nigeria [23] were 3% and 1% compared to 13.1% and 1.4% seen respectively in the present study. In another study done in Thailand, FPR for detection of Plasmodium vivax, ovale and malariae were found to be 25.9% and for Plasmodium falciparum was found to be 60.3% using MRDT kits [32].
In the present study, DOR was reported to be 470.6. DOR is dependent on the spectrum of disease severity [33], is independent of disease prevalence and is a measure of the effectiveness of a diagnostic test [26,34]. Higher the DOR, better is the test performance [26]. The high DOR values observed in this study was due to high positive diagnostic and low negative DLRs which were found to be 7.53 and 0.016 respectively. Another study done in Ahmedabad, India [22], reported a positive likelihood ratio of 11.7, a negative likelihood ratio of 0.013 and DOR of 900. A study done in Ghana reported DOR of even 2366.4 [26]. A very high value of positive likelihood ratio confirms that patients with positive tests with MRDTs have a high probability of being infected. Similarly, very low values for negative likelihood ratio confirms that patients with negative test have a high probability of not having infection. DLR is more clinically useful than the sole usage of sensitivity or specificity in estimating the probability of disease in an individual [35].
In the study done in Ghana [26], FDR was 2% and FOR was 2% in comparison to 5.9% and 3.4% respectively reported in this study. FDR and FOR procedures were designed to complement PPV and NPV respectively; hence lower the FDR and FOR values better is the performance of the diagnostic test [26].
For Plasmodium vivax cases, in particular, sensitivity of RDT was 98.9% in the present study compared to 97.3% [36] and 98.6% [22] observed in other studies and specificity was 86.9% in the present study compared to 90% [22], 98.7% [36] and 99% [37] reported in previous studies. The PPV for Plasmodium vivax malaria was 93.2% in the present study in comparison to 52.7% [22] and 94.7% [36] reported in previous studies and NPV was 97.7% in the present study in comparison to 99.3% [36] and 99.8% [22] reported respectively in previous studies.
For Plasmodium falciparum cases in particular, sensitivity of RDT was 100% in the present study compared to 94.2% [36] and 100% [22,37] reported in other studies and specificity was 86.9% in the present study compared to 97.3% [22] and 99.5% [36] reported respectively in previous studies. The PPV for Plasmodium falciparum malaria was 61.8% in the present study compared to 52.7% [22] and 99.3% [36] reported in previous studies and NPV was 100% in the present study compared to 96% [36] and 100% [22] reported respectively in previous studies. The present study, therefore, observed that MRDT has a higher sensitivity and higher NPV whereas lower PPV for diagnosis of falciparum compared to vivax malaria. Similar observations were made in another study done in France [37].
In the present study, sensitivity and specificity of RDTs in diagnosing malaria was higher among younger patients in comparison to adults. Previous studies have also reported that sensitivity of MRDTs was higher in younger age groups [17,39]. On the contrary, a study done in Nigeria reported that sensitivity of RDT was found to increase with age of the participants with values as 33.3%, 41.2% and 42.6% recorded in school children, teenagers and adult patients respectively [24]. The AUC value in the present study was 0.927. AUC values indicate the probability of the test to correctly categorise the patients as true positives or negatives. In other words, it is a measure of discriminating ability of the test [40]. Higher the values, better is the diagnostic test. Value of AUC observed in the present study was significantly higher than 0.5 and hence, MRDT is a good test for predicting the outcome. On the other hand, AUC value of 0.5 implies poor predictive power or no agreement between tests [40].
Overall, antigen detecting MRDT kits showed good performance as a screening test. Unlike the antibody detecting MRDT kits, which have been banned for usage for malaria screening purposes by the Union Health Ministry, Government of India due to its poor specificity. This was because antibody detecting MRDT kits were testing positive for antibodies which persisted even after clearance of active malaria infections leading to high FPR [41,42].
Limitation
The malarial parasite load of patients which can also influence the validity of MRDTs was not assessed in the present study. Exposure of RDT kit to high temperatures in the tropics results in denaturation of antibodies in the test membrane. This can impair binding to the target antigen at high temperature. Heat can also cause damage to the nitrocellulose membrane forming the strip thus changing its flow characteristics or causing the antibody to detach from the membrane. Other factors like high humidity can rapidly degrade nitrocellulose capillary flow action in MRDT kits. As Mangalore has a typical tropical climate, health care providers should take adequate precautions in the storage of RDT kits to ensure quality.
Conclusion
Antigen detecting MRDT kits showed good performance as a screening test. Therefore it can be recommended for wide-scale usage at this settings. Deployment of RDTs can also potentially substitute microscopy services in resource constrained settings. However, adequate surveillance is required, to ensure maintenance of quality of kits in different lots, both during manufacturing and post-marketing stages, considering the warm and humid climatic conditions in Mangalore, India.
*Peripheral smear, **Rapid diagnostic test