Introduction
Developing country like India has shown a rising trend in deaths related to pancreatic carcinoma and is mainly associated with rapid urbanization and increased alcohol consumption. Early detection plays an important role in surgical management. Imaging has played a crucial role and in a resource poor setting like India they are even more important. This review highlights various imaging techniques, their findings and the comparative statistical analysis.
Imaging
Conventional Radiography
Before the advent of cross-sectional imaging, imaging of the pancreas was largely limited to radiographic identification of pancreatic calcification and it was used to diagnose various pancreatic diseases. Pancreatic calcifications have been largely associated with chronic calcific pancreatitis. Although alcohol abuse remains the predominant cause of pancreatic calcifications, many other conditions can also show calcification. About 20-40% of cases with chronic alcoholic pancreatitis show radiographically visible pancreatic calcification and it is seen to develop in a course of about 5-10 years [1]. Ductal adenocarcinoma characteristically does not calcify, however progressive displacement of calcium on serial films can provide a clue to the presence of an enlarging tumour. Rarely, calcification disappears with the development of pancreatic cancer.
Cystadenoma or cystadenocarcinoma show calcification with the sunburst pattern being pathognomonic which on gross pathologic finding reflects multiple cystic spaces separated by spoke-like stromal elements that radiate from a central nidus in about 10% cases [1]. Islet cell tumours are known for the presence of tumoural calcifications which appears as focal, coarse, irregular calcification noted within the mass. Insulinoma, which is the most common, can contain calcifications in up to 20% of cases [2]. Solid and pseudopapillary epithelial neoplasms also show calcification which is usually punctate and peripherally located. Pancreatoblastoma, can develop calcifications in up to 20% of cases [3]. Because of several overlapping causes of pancreatic calcification, the sensitivity of conventional radiography is about 30-70% [4].
Barium Study
Barium examination of the upper gastrointestinal tract and duodenum provides indirect information of pancreatic pathology. Although the duodenal changes produced by pancreatic disease may be detected by the conventional barium examination, however, the large number of false-positive and false-negative studies led to the use of techniques developed specifically for examination of the duodenum like tube-assisted duodenography and double-contrast duodenography [5].
Masses in the head of the pancreas typically cause impressions on either the stomach or the duodenal C-loop resulting in antral pad sign when indenting the greater curvature of stomach and double-contour effect when against the inner aspect of the duodenum. Localized impressions on the C-loop can cause nodular indentations. Malignant disease infiltrating the wall of the duodenum can produce an inverted-3 appearance or Frostberg’s sign; this is a nonspecific sign, which is seen in less than 10% of patients with pancreatic carcinoma [1]. Enlarging pancreatic mass sometimes causes change/distortion in the duodenal diverticula. Fine or coarse spiculations are formed by barium filled crevices between duodenal plical folds and are secondary to mucosal oedema and can be seen in pancreatitis or pancreatic carcinoma. Both pancreatic carcinoma and chronic pancreatitis can present with mucosal flattening, fold effacement and a slight reduction in luminal caliber, however, duodenal ulceration and obstruction favours more towards pancreatic carcinoma. Enlargement of lymph nodes near the head of the pancreas can widen the duodenal C-loop [6,7].
Conventional Angiography
Tillander was one of the first angiographers to perform selective arteriography specifically for evaluation of the pancreas and later it was Odman, Olsson, and Boijsen in Sweden and Rösch and Bret in Czechoslovakia who pioneered many of the pancreatic angiography techniques. The accuracy of angiography in the diagnosis of pancreatic carcinoma depended mainly on the techniques employed and it ranged from 29 to 75% [8,9].
Endoscopic Retrograde Cholangiopancreatography (ERCP)
This technique is ideally suited for early detection of pancreatic adenocarcinoma because adenocarcinoma arises from the ductal system. The main findings which are seen on ERCP include ductal obstruction or encasement, acinar defects, and tumour cavities that communicate with the pancreatic duct. Earlier publications have shown a sensitivity of 94%, specificity of 97% [10]. Brush cytology when performed along with ERCP has 35-70% sensitivity and 90% specificity [11]. Triple sampling using brush cytology, FNA and forceps biopsy of biliary stricture during ERCP improves the sensitivity for diagnosing cancer to 77% [12].
Trans-Abdominal Ultrasound
Filly RA et al., in 1970 were the first to report the use of ultrasound for the pancreas using Ultrasonic laminagraph [13]. Duplex Doppler sonography was initially used for the assessment of portal venous system in portal hypertension [14]. Garber and Lees in 1992 reported a change in portal vein velocity caused by tumour compression or ingrowth [15]. The most common ultrasonographic manifestation of pancreatic carcinoma is a focal or diffuse lesion. Lesions >2 cm in diameter are better seen on ultrasonography. A specific diagnosis of neoplasm can be made if additional findings such as hepatic metastases or regional adenopathy are seen. Pancreatic carcinoma appears hypoechoic when compared to the normal pancreas. Rarely hyperechoic lesions have also been noted. The normal pancreatic duct has smooth, parallel echoic walls with a normal diameter of less than 2 mm.
One of the chief advantages of ultrasonography is the ability to evaluate the liver and biliary ducts. About 47% of patients with pancreatic carcinoma have liver metastases at the time of initial evaluation [16]. Abdominal ultrasound is often the first line examination for a patient presenting with jaundice and pain. The reported accuracy of ultrasound for detecting pancreatic lesion is around 87.8% [17]. Colour Doppler has a sensitivity of 60%-90% for detection of vascular invasion [18]. The sensitivity of transabdominal ultrasound depends on patient-dependent factors such as overlying bowel gas, obesity, patient co-operation, operator variability, ultrasound equipment used, and the size and location of the tumour.
Contrast-Enhanced Ultrasonography
Rickes et al., reported lesion detection rate of 87% against 57% in non-contrast studies [19]. Pancreatic adenocarcinoma is hypovascular, whereas endocrine cell tumours are mostly hypervascular and pancreatitis-associated mass are mostly isovascular. Kitano M et al., assessed the role of harmonic imaging and found a sensitivity and specificity of 90% and 95% respectively in the detection of pancreatic ductal carcinoma [20].
Laparoscopic Ultrasonography (LUS)
This technique was first reported by Fukuda M et al., in 1984 [21]. High frequency LUS probes enable the on table evaluation of various solid organs, retroperitoneal space, lymph node, peritoneal deposits, and vascular infiltration with a higher resolution. In a study by Bemelman WA et al., laparoscopy when combined with US showed a positive predictive value of 97% for unresectability [22]. Unnecessary laparotomy could be prevented in at least 40% of patients with the use of LUS [23]. The disadvantage of LUS includes difficulty in the visualization of a local tumour extension in major vessels, sonographically-guided biopsies, and a long learning curve.
Intravascular Ultrasonography (IVUS)
IVUS is a technique to detect intraportal thrombus which can sometimes be missed on CT and is performed either by trans-hepatic or trans-mesenteric route. Kaneko T et al. pioneered the use of IVUS in the staging of pancreatic cancer [24]. IVUS has been reported only in the evaluation of portal and superior mesenteric vein and has shown a sensitivity of >95% and specificity of >90% [24,25]. IVUS is superior to CT and portography for the detection of venous invasion.
Endoscopic Ultrasonography (EUS)
EUS was first reported by DiMagno EP et al., for the visualization of the pancreas using a mechanical radial endoscopic ultrasound system [26]. EUS is good for detection <20 mm lesions, with a sensitivity of 93-100% [27]. EUS guided FNAC was first described in 1990 and is found to have a sensitivity and specificity of 86.8% and 95.8%, respectively [28]. Newer techniques have been introduced, the diagnostic yield of EUS which includes Contrast enhanced EUS and EUS Elastography. Contrast-enhanced harmonic EUS has shown a sensitivity and specificity of 95.1% and 89% respectively [29]. The limitations of EUS are operator dependence, limited experience, and inability to examine the entire liver and peritoneal metastases. Complications of EUS include pancreatitis, bleeding, tear, and anaesthetic complications.
Computed Tomography (CT)
The use of CT in the pancreas was first reported by Haaga JR et al., in 1976 with a study group of 82 patients [30]. Pancreatic tumours are visible on CT as focal and abrupt changes in size or contour of the organ. Adenocarcinomas present as hypo-enhancing lesion in the background of normal pancreatic parenchyma [Table/Fig-1,2]. Pancreatic duct can either present as a focal narrowing or as an apparent disappearance known as the “missing duct sign”. Pancreatic head mass is associated with “Double duct sign” i.e., dilated pancreatic duct and common bile duct. Small areas of necrosis are seen as hypodense foci located within the mass. Occasionally, adenocarcinomas may contain large cystic or necrotic areas. Tumours like serous or mucinous tumours appear predominantly cystic.
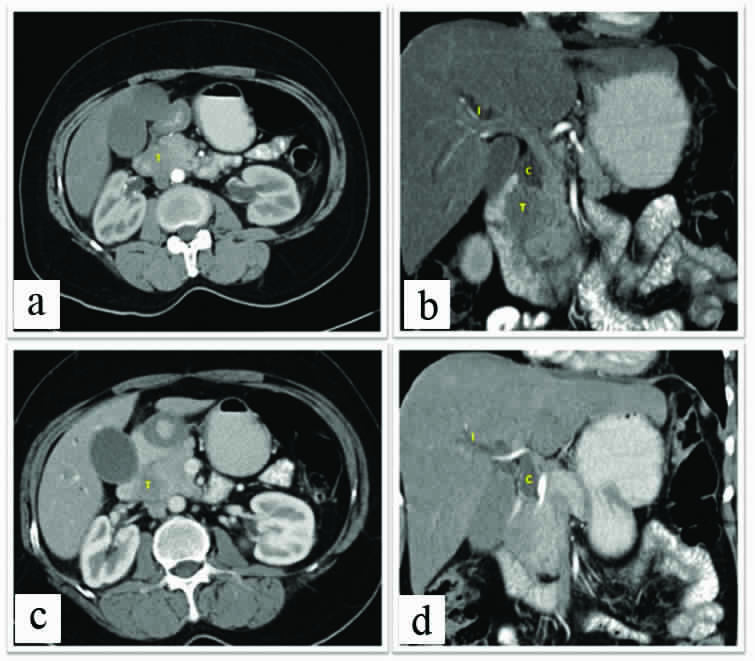
Contrast enhanced CT, Images a and c: Axial sections and Images b and d: reformatted Coronal Images, Shows pancreatic lesion “T” well visualised in pancreatic head with isolated CBD dilatation (C) andIHBRD (Intra Hepatic Biliary Duct Dilatation) (I). The lesion shows loss of fat plane with duodenum.
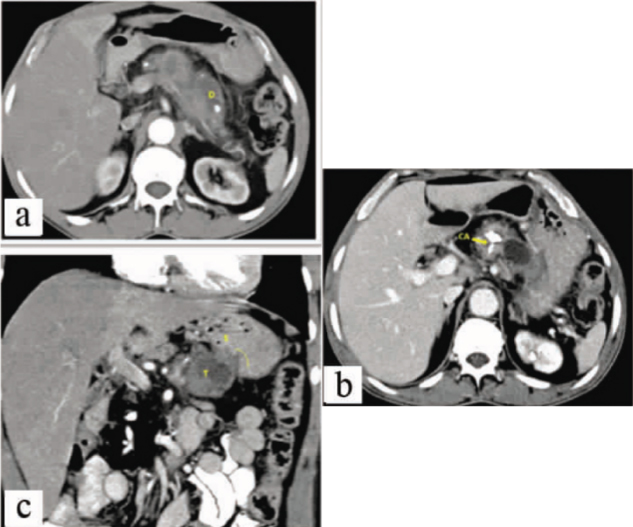
Contrast enhanced CT, Images a and b: Axial sections and Images c Reformatted Coronal Images, Shows pancreatic lesion “T” well visualised in pancreatic body with the loss of fat plane and local infiltration (Yellow line) into stomach (S) and Pancreatic Duct dilatation (D). Pancreas shows Parenchymal and ductal calcification (CA) suggestive of background chronic calcific pancreatitis.
Indirect CT signs are secondary to ductal obstruction. Pancreatic duct dilatation is an important feature in ductal adenocarcinoma. Associated dilatation of side branches is also a common finding. Post-obstructive pseudocysts are seen in about 10% of patients [31]. Biliary dilatation is frequently present in ductal carcinoma of the pancreatic head due to the direct involvement of the intrapancreatic portion of the common bile duct. Intrahepatic duct dilatation [Table/Fig-1] is considered to be present when the intrahepatic ducts are visible in the peripheral portion of the liver, or if the diameter exceeds 4 mm in the region of the liver hilum. Gallbladder distension is most often associated with bile duct dilatation, if the level of the biliary obstruction is distal to the junction of the cystic duct. Liver metastases are seen as hypo enhancing lesions better appreciated on the hepatic phase of perfusion and are usually not as well seen in pancreatic phase. Peritoneal metastasis is not uncommon in carcinoma pancreas. CT allows for earlier detection of enlarged lymph nodes; however, it does not allow differentiation between benign and malignant causes of lymph node enlargement. CT is currently well established as the primary imaging modality for diagnosis and staging of pancreatic cancer.
Toft et al., in a meta-analysis found a sensitivity, specificity and accuracy of 90%, 87% and 89% respectively for the diagnosis of pancreatic adenocarcinoma [32]. Zamboni GA et al., compared sensitivity and specificity of 4, 8, 16 and 64 slice scanners and found almost similar sensitivity and specificity with no significant variation in results using various scanners [33].
Recent advances in CT have taken place with the advent of Dual-energy CT and low-tube-voltage techniques. The detection of small pancreatic cancers <2 cm in diameter or of isoattenuating tumours remains a challenge when diagnosing using MDCT. Dual-energy technique improves the contrast-to-noise ratio between pancreatic cancer and normal parenchyma. A low-tube-voltage CT technique increases the X-ray absorption of iodine by increasing k edge effect of iodine resulting in improved contrast enhancement of normal pancreatic parenchyma and delineating the poorly vascularized pancreatic cancers [34,35]. A new method to reduce CT noise is based on an Iterative Reconstruction (IR) algorithm which has recently been used for higher resolution image production.
Magnetic Resonance Imaging
Smith FW et al., first reported the use of nuclear magnetic imaging technique for pancreas in 1982 using a 0.004 Tesla scanner, with two cases out of 12 cases suggestive of pancreatic carcinoma [36]. Semelka RC et al., in 1993 demonstrated that pancreas could be best visualized with T1-weighted breath hold gradient-echo imaging, which avoids phase artifact caused by respiration, and T1-weighted fat-suppressed spin-echo imaging, which reduces breathing artifact, removes chemical shift artifact and improves the dynamic range of signal intensities [37]. The normal pancreas has higher signal intensity due to the abundance of protein and rough endoplasmic reticulum within it. Fat suppression on T1-weighted images renders the normal pancreas high in signal intensity due to the presence of aqueous protein, and the pancreatic tumour stands out. Pancreatic masses are best visualized and evaluated using a combination of unenhanced and early gadolinium-enhanced T1-weighted sequences. The venous and delayed phases of gadolinium enhanced sequences are best for detecting peripancreatic and periportal lymphadenopathy as well as peritoneal metastases. Toft J et al. in a meta-analysis found a sensitivity, specificity and accuracy of 93%, 89% and 90% respectively for the diagnosis of pancreatic adenocarcinoma [32]. When comparing MRI, EUS and CT, studies have shown that MRI is very good for staging and suggesting arterial involvement but has a lower accuracy rate [38].
MRCP (Magnetic Resonance Cholangiopancreatography)
A 3D TSE sequence produces high spatial-resolution MRCP images as thinner sections without a slice gap, allow better assessment of small stones, side branches of the main pancreatic duct, and intrahepatic bile ducts. MRCP is a non-invasive procedure with almost equal diagnostic importance as ERCP. The only advantage ERCP has over MRCP is that both diagnostic and a therapeutic maneuver can be attempted together. Hekimoglu K et al., compared ERCP over MRCP and found that MRCP was 100% sensitive and specific in diagnosing pancreatic tumours [39].
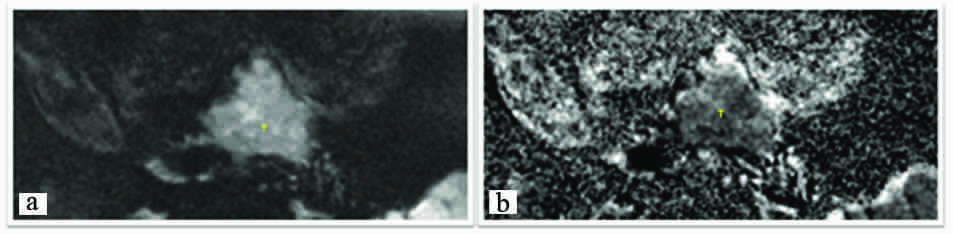
Qualitative analysis: Images a and b: Axial Images, Diffusion Weighted Image (b value- 500) shows lesion (T) which shows diffusion restriction with low signals on ADC mapping.
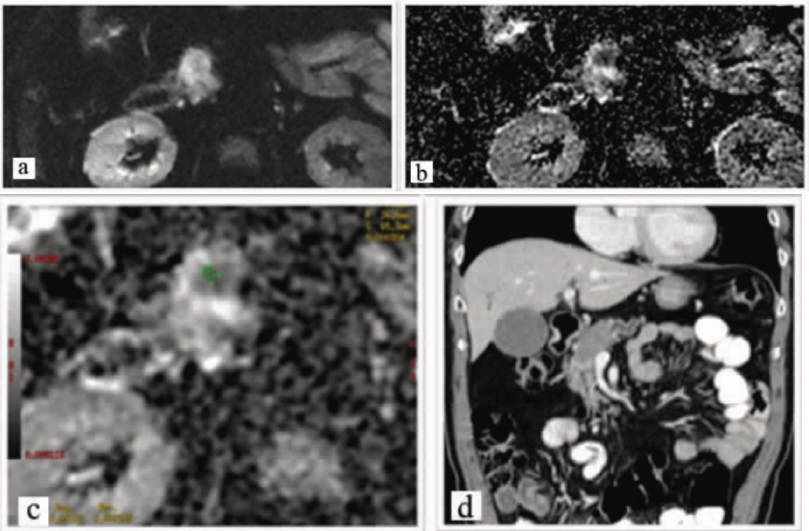
Quantitative analysis: Images a, b and c: Axial Images, Diffusion Weighted Image (b value- 500) shows lesion which shows diffusion restriction with low signals with low ADC value on ADC mapping. Image d shows coronal reformatted image which shows the hypodense lesion with pancreatic duct dilatation duct.
Recently it has shown promising results when used in the pancreatic evaluation. Chronic pancreatitis can be challenging and confusing even on DWI. Visual assessment of DWI can be misleading in chronic pancreatitis as suggested by Fukukura Y et al., [40]. Mean ADC value for chronic pancreatitis was 1.24±0.23×10-3 mm2/s, p=0.004 and that for pancreatic adenocarcinoma was 1.160±0.22×10-3 mm2/s [40]. Fattahi R et al., showed ADC values of 1.46×10-3 mm2/s in case of carcinoma pancreas which was lower than those of the normal pancreatic parenchyma 2.11×10-3mm2/s [41].
Advances in MR technology have caused great improvements in pancreatic cancer imaging. Several literature reports have described the comparable diagnostic performance of MDCT and MR. Koelblinger C et al., reported the mean sensitivity and specificity of 95% vs. 96%, and 96% respectively when comparing 64-detector row CT and MR for the detection of pancreatic cancer [42]. In quantitative analysis of dynamic contrast enhanced-MR imaging the enhancement patterns and perfusion parameters are analyzed, and this has shown to be both objective and helpful in the evaluation of malignant diseases regarding both their diagnosis and treatment monitoring. The K(trans), K(ep), and iAUC values is lower in patients with pancreatic cancer compared to normal pancreas. The Intravoxel Incoherent Motion (IVIM) model provides a theoretical framework from which to derive diffusion and perfusion parameters from DWI. Recently, the IVIM approach with multiple b-values has been applied to pancreatic imaging, and there have been several reports showing promising results regarding the differentiation of pancreatic cancer from normal pancreas. Gadoxetic acid-enhanced liver MR is 85% sensitive for detecting liver metastasis in pancreatic cancer, which is significantly higher compared with that of CT which is about 69% [43]. Integrated PET and MR (PET/MR) scanners have recently become available, and as MR has the inherent strength of superior soft-tissue contrast resolution, multiplanar imaging acquisition, and functional imaging capability, its diagnostic performance is superior compared with that of PET/CT.
Positron Emission Tomography (PET)
A 18-Fluorodeoxyglucose (FDG) provides an alternative approach to the diagnosis of pancreatic cancer. A malignant cell has an increased rate of glycolysis compared with that of a normal cell. FDG is a glucose analog that is transported into cells by an uptake mechanism similar to that by which glucose is transported. The expression of the glucose transporter gene and other glycolysis-associated genes is increased compared with that found in a normal pancreas, which is likely to contribute to increased metabolic capacity. These increases in gene expression provide a mechanism for further accumulation of FDG in patients with pancreatic cancer [44]. The accumulation of FDG in infectious or benign inflammatory conditions has been well documented as a cause of false-positive PET studies. False-negative studies have been reported with small (<2 cm) tumours and in diabetes mellitus. PET is a crucial imaging technique to assess peripancreatic lymphadenopathy.
Reports have suggested that PET may have an advantage over CT in showing the local nodal spread in pancreatic cancer. Sensitivity and specificity after a positive CT was 87-95% and 51-81%; after a negative CT, the corresponding values were 50-88% and 75-93% [45]. Recent studies have evaluated the value of integrated PET/CT, which has a better spatial resolution as compared to PET scans. Pancreatic adenocarcinoma has a Standardized Uptake Value of 3.50±1.66 which is higher than both benign lesions (1.91±0.65) and the normal pancreas [46]. In one case series, the sensitivity and specificity of PET/CT was found to be 89% and 69% respectively [47]. PET/CT is also superior to conventional MDCT used for tumour staging and detection of distant metastases (with sensitivity and specificity rates were 89 versus 56 and 100 versus 95%, respectively) [48]. Another study found PET to have comparable sensitivity with MRI but had lower sensitivity compared to MRI and EUS [48].
Conclusion
Imaging plays a crucial role in the diagnosis and management of carcinoma pancreas. Each modality has its pros and cons and a multimodality approach aids in diagnosis, management and follow up of these patients. In a resource poor setting, earlier imaging modalities can help in arrival at a stronger suspicion which can then be referred to a higher centre for further evaluation.
[1]. Eisenberg R, Gastrointestinal radiology 1996 4th editionPhiladelphiaLippincott-Raven Publishers:345-347. [Google Scholar]
[2]. Buetow PC, Parrino TV, Buck JL, Pantongrag-Brown L, Ros PR, Dachman AH, Islet cell tumours of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional statusAJR 1995 165(5):1175-79.10.2214/ajr.165.5.75724987572498 [Google Scholar] [CrossRef] [PubMed]
[3]. Montemarano H, Lonergan GJ, Bulas DI, Selby DM, Pancreatoblastoma: imaging findings in 10 patients and review of the literatureRadiology 2000 214(2):476-82.10.1148/radiology.214.2.r00fe3647610671596 [Google Scholar] [CrossRef] [PubMed]
[4]. Perez-Johnston R, Sainani NI, Sahani DV, Imaging of chronic pancreatitis (including groove and autoimmune pancreatitis)Radiol Clin North Am 2012 50(3):447-66.10.1016/j.rcl.2012.03.00522560691 [Google Scholar] [CrossRef] [PubMed]
[5]. Bilbao MK, Frische LH, Dotter CT, Rösch J, Hypotonic duodenographyRadiology 1967 89(3):438-43.10.1148/89.3.4386034912 [Google Scholar] [CrossRef] [PubMed]
[6]. Macchia B, Bobruff J, Groissier VW, Positional relief of pain; important clue to clinical diagnosis of carcinoma of the pancreasJAMA 1962 182:6-10.10.1001/jama.1962.0305040000800214467766 [Google Scholar] [CrossRef] [PubMed]
[7]. Gambill EE, Pancreatitis associated with pancreatic carcinoma: a study of 26 casesMayo Clinic Proceedings 1971 46:174-77. [Google Scholar]
[8]. Mackie CR, Noble HG, Cooper MJ, Collins P, Block GE, Moossa AR, Prospective evaluation of angiography in the diagnosis and management of patients suspected of having pancreatic cancerAnn Surg 1979 189(1):11-17.10.1097/00000658-197901000-00003758855 [Google Scholar] [CrossRef] [PubMed]
[9]. Nebesar RA, Pollard JJ, A critical evaluation of selective celiac and superior mesenteric angiography in the diagnosis of pancreatic disease, particularly malignant tumour: Facts and “artifacts.”Radiology 1967 89:1017-27.10.1148/89.6.10174293858 [Google Scholar] [CrossRef] [PubMed]
[10]. Freeny PC, Ball TJ, Endoscopic Retrograde Cholangiopancreatography (ERCP) and Percutaneous Transhepatic Chalangiography (PTC) in the evaluation of suspected pancreatic carcinoma: Diagnostic limitations and contemporary rolesCancer 1981 47(6):1666-78.10.1002/1097-0142(19810315)47:6+<1666::AID-CNCR2820471435>3.0.CO;2-U [Google Scholar] [CrossRef]
[11]. Trent V, Khurana KK, Pisharodi LR, Diagnostic accuracy and clinical utility of endoscopic bile duct brushing in the evaluation of biliary stricturesArch Pathol Lab Med 1999 123(8):712-15. [Google Scholar]
[12]. Jailwala J, Fogel EL, Sherman S, Gottlieb K, Flueckiger J, Bucksot LG, Triple-tissue sampling at ERCP in malignant biliary obstructionGastrointest Endosc 2000 51(4pt 1):383-90.10.1016/S0016-5107(00)70435-4 [Google Scholar] [CrossRef]
[13]. Filly RA, Freimanis AK, Echographic diagnosis of pancreatic lesions. Ultrasound scanning techniques and diagnostic findingsRadiology 1970 96(3):575-82.10.1148/96.3.5755468845 [Google Scholar] [CrossRef] [PubMed]
[14]. Angeli E, Venturini M, Vanzulli A, Sironi S, Castrucci M, Salvioni M, Color Doppler imaging in the assessment of vascular involvement by pancreatic carcinomaAJR Am J Roentgenol 1997 168(1):193-97.10.2214/ajr.168.1.89769458976945 [Google Scholar] [CrossRef] [PubMed]
[15]. Garber SJ, Lees WR, The characterization of pancreatic and bile duct tumours by duplex DopplerClin Radiol 1992 45:181-84.10.1016/S0009-9260(05)80637-3 [Google Scholar] [CrossRef]
[16]. Sullivan DC, Taylor KJW, Gottschalk A, The use of ultrasound to enhance the diagnostic utility of the equivocal liver scintigraphRadiology 1978 128(3):727-32.10.1148/128.3.727674646 [Google Scholar] [CrossRef] [PubMed]
[17]. D’Onofrio M, Barbi E, Dietrich CF, Kitano M, Numata K, Sofuni A, Pancreatic multicenter ultrasound study (PAMUS)Eur. J. Radiol 2012 81(4):630-38.10.1016/j.ejrad.2011.01.05321466935 [Google Scholar] [CrossRef] [PubMed]
[18]. Casadei R, Ghigi G, Gullo L, Moretti CC, Greco VM, Salizzoni E, Role of color Doppler ultrasonography in the preoperative staging of pancreatic cancerPancreas 1998 16(1):26-30.10.1097/00006676-199801000-000059436859 [Google Scholar] [CrossRef] [PubMed]
[19]. Rickes S, Unkrodt K, Neye H, Okran KW, Wermke W, Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonographyScand J Gastroenterol 2002 37:1313-20.10.1080/00365520276102060512465731 [Google Scholar] [CrossRef] [PubMed]
[20]. Kitano M, Kudo M, Maekawa K, Suetomi Y, Sakamoto H, Fukuta N, Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography 2004 53(6):854-59.10.1136/gut.2003.02993415138213 [Google Scholar] [CrossRef] [PubMed]
[21]. Fukuda M, Mirna S, Tanabe T, Hanui T, Suzuki Y, Hirata K, Endoscopic sonography of the liver-diagnostic application of the echolaparoscope to localize intrahepatic lesionsScand J Gastroentero 1984 102:24-38. [Google Scholar]
[22]. Bemelman WA, de Wit LT, van Delden OM, Smits NJ, Obertop H, Rauws EAJ, Diagnostic laparoscopy combined with laparoscopic ultrasonography in staging of cancer of the pancreatic head regionBr J Surg 1995 82(6):820-24.10.1002/bjs.18008206337627522 [Google Scholar] [CrossRef] [PubMed]
[23]. Van Delden OM, de Wit LT, Bemelman WA, Reeders JW, Gouma DJ, Laparoscopic ultrasonography for abdominal tumor staging: technical aspects and imaging findingsAbdom Imaging 1997 22(2):125-31.10.1007/s0026199001569013519 [Google Scholar] [CrossRef] [PubMed]
[24]. Kaneko T, Nakao A, Inoue S, Harada A, Nonami T, Itoh S, Intraportal endovascular ultrasonography in the diagnosis of portal vein invasion by pancreatobiliary carcinomaAnn Surg 1995 222(6):711-18.10.1097/00000658-199512000-000048526577 [Google Scholar] [CrossRef] [PubMed]
[25]. Kaneko T, Nakao A, Inoue S, Endo T, Itoh S, Harada A, Portal venous invasion by pancreatobiliary carcinoma: diagnosis with intraportal endovascular USRadiology 1994 192(3):681-86.10.1148/radiology.192.3.80589338058933 [Google Scholar] [CrossRef] [PubMed]
[26]. DiMagno EP, Buxton JL, Regan PT, Hattery RR, Wilson DA, Suarez JR, Ultrasonic endoscopeLancet 1980 1(8169):629-31.10.1016/S0140-6736(80)91122-8 [Google Scholar] [CrossRef]
[27]. Kahl S, Glasbrenner B, Zimmermann S, Malfertheiner P, Endoscopic ultrasound in pancreatic diseasesDig Dis 2002 20(2):120-26.10.1159/00006748112566614 [Google Scholar] [CrossRef] [PubMed]
[28]. Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA, How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic reviewPancreas 2013 42(1):20-26.10.1097/MPA.0b013e3182546e7923254913 [Google Scholar] [CrossRef] [PubMed]
[29]. Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Characterization of smallsolid tumours in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonographyAm J Gastroenterol 2012 107(2):303-10.10.1038/ajg.2011.35422008892 [Google Scholar] [CrossRef] [PubMed]
[30]. Haaga JR, Alfidi RJ, Zelch MG, Meany TF, Boller M, Gonzalez L, Computed tomography of the pancreasRadiology 1976 120:589-95.10.1148/120.3.589781727 [Google Scholar] [CrossRef] [PubMed]
[31]. Lin JT, Wang TH, Chen DS, How SW, Lai MY, Chen JC, Pancreatic carcinoma associated with chronic calcifying pancreatitis in Taiwan: a case report and review of literaturePancreas 1988 3(1):111-14.10.1097/00006676-198802000-000203283730 [Google Scholar] [CrossRef] [PubMed]
[32]. Toft J, Hadden WJ, Laurence JM, Lam V, Yuen L, Janssen A, Imaging modalities in the diagnosis of pancreatic adenocarcinoma: A systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracyEuropean Journal of Radiology 2017 92:17-23.10.1016/j.ejrad.2017.04.00928624015 [Google Scholar] [CrossRef] [PubMed]
[33]. Zamboni GA, Kruskal JB, Vollmer CM, Baptista J, Callery MP, Raptopoulos VD, Pancreatic adenocarcinoma: value of multidetector ct angiography in preoperative evaluationRadiology 2007 245(3):770-78.10.1148/radiol.245306179517951353 [Google Scholar] [CrossRef] [PubMed]
[34]. Marin D, Nelson RC, Barnhart H, Schindera ST, Ho LM, Jaffe TA, Detection of pancreatic tumours, image quality, and radiation dose during the pancreatic parenchymal phase: effect of a low tube-voltage, high-tube-current CT technique--preliminary resultsRadiology 2010 256(2):450-59.10.1148/radiol.1009181920656835 [Google Scholar] [CrossRef] [PubMed]
[35]. Heye T, Nelson RC, Ho LM, Marin D, Boll DT, Dual-energy CT applications in the abdomenAJR Am J Roentgenol 2012 199(5):S64-S70.10.2214/AJR.12.919623097169 [Google Scholar] [CrossRef] [PubMed]
[36]. Smith FW, Reid A, Hutchinson JMS, Mallard JR, Nuclear magnetic resonance imaging of the pancreasRadiology 1982 142(3):677-80.10.1148/radiology.142.3.70636837063683 [Google Scholar] [CrossRef] [PubMed]
[37]. Semelka RC, Kroeker MA, Shoenut JP, Kroeker R, Yaffe CS, Micflikier AB, Pancreatic disease: prospective comparison of CT, ERCP, and 1.5-T MR imaging with dynamic gadolinium enhancement and fat suppressionRadiology 1993 181(3):785-91.10.1148/radiology.181.3.19470981947098 [Google Scholar] [CrossRef] [PubMed]
[38]. Mehmet Erturk S, Ichikawa T, Sou H, Saitou R, Tsukamoto T, Motosugi U, Pancreatic adenocarcinoma: MDCT versus MRI in the detection and assessment of locoregional extensionJ Comput Assist Tomogr 2006 30(4):583-90.10.1097/00004728-200607000-0000616845288 [Google Scholar] [CrossRef] [PubMed]
[39]. Hekimoglu KI, Ustundag Y, Dusak A, Erdem Z, Karademir B, Aydemir S, MRCP vs. ERCP in the evaluation of biliary pathologies: review of current literatureJ Dig Dis 2008 9(3):162-69.10.1111/j.1751-2980.2008.00339.x18956595 [Google Scholar] [CrossRef] [PubMed]
[40]. Fukukura Y, Takumi K, Kamimura K, Shindo T, Kumagae Y, Tateyama A, Pancreatic adenocarcinoma: variability of diffusion-weighted MR imaging findingsRadiology 2012 263(3):732-40.10.1148/radiol.1211122222623694 [Google Scholar] [CrossRef] [PubMed]
[41]. Fattahi R, Balci NC, Perman WH, Hsueh EC, Alkaade S, Havlioglu N, Pancreatic Diffusion-Weighted Imaging (DWI): comparison between mass-forming focal pancreatitis (FP), Pancreatic Cancer (PC), and normal pancreasJ Magn Reson Imaging 2009 29(2):350-56.10.1002/jmri.2165119161187 [Google Scholar] [CrossRef] [PubMed]
[42]. Koelblinger C, Ba-Ssalamah A, Goetzinger P, Puchner S, Weber M, Sahora K, Gadobenate dimeglumine-enhanced 3.0-T MR imaging versus multiphasic 64-detector row CT: prospective evaluation in patients suspected of having pancreatic cancerRadiology 2011 259(3):757-66.10.1148/radiol.1110118921436084 [Google Scholar] [CrossRef] [PubMed]
[43]. Motosugi U, Ichikawa T, Morisaka H, Sou H, Muhi A, Kimura K, Detection of pancreatic carcinoma and liver metastases with gadoxetic acid-enhanced MR imaging: comparison with contrast-enhanced multi-detector row CTRadiology 2011 260(2):446-53.10.1148/radiol.1110354821693662 [Google Scholar] [CrossRef] [PubMed]
[44]. Persons DA, Schek N, Hall BL, Finn OJ, Increased expression of glycolysis-associated genes in oncogene-transformed and growth-accelerated statesMol Carcinog 1989 2(2):88-94.10.1002/mc.29400202072765128 [Google Scholar] [CrossRef] [PubMed]
[45]. Sendler A, Avril N, Helmberger H, Stollfuss J, Weber W, Bengel F, Preoperative evaluation of pancreatic masses with positron emission tomography using 18Ffluorodeoxyglucose: diagnostic limitationsWorld J Surg 2000 24(9):1121-29.10.1007/s00268001018211036292 [Google Scholar] [CrossRef] [PubMed]
[46]. Koyama K, Okamura T, Kawabe J, Nakata B, Chung KH, Ochi H, Diagnostic usefulness of FDG PET for pancreatic mass lesionsAnn Nucl Med 2001 15(3):217-24.10.1007/BF0298783511545191 [Google Scholar] [CrossRef] [PubMed]
[47]. Heinrich S, Goerres GW, Schäfer M, Sagmeister M, Bauerfeind P, Pestalozzi BC, Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectivenessAnn Surg 2005 242(2):235-43.10.1097/01.sla.0000172095.97787.8416041214 [Google Scholar] [CrossRef] [PubMed]
[48]. Borbath I, Van Beers BE, Lonneux M, Schoonbroodt D, Geubel A, Gigot JF, Preoperative assessment of pancreatic tumours using magnetic resonance imaging, endoscopic ultrasonography, positron emission tomography and laparoscopyPancreatology 2005 5(6):553-61.10.1159/00008749716113592 [Google Scholar] [CrossRef] [PubMed]