Introduction
Momordica charantia (Bitter Melon) is known to have anti-diabetic property. Animal studies have documented its hypoglycaemic and lipid lowering effect. However, clinical trials with human subjects are very few and the effective dose of Momordica charantia is not studied in type-2 diabetes mellitus patients.
Aim
To investigate the effect of Momordica charantia (1 gm and 1.5 gm) on glycaemic profile, insulin resistance, lipid profile, oxidative stress and Body Mass Index (BMI) in type-2 diabetes mellitus patients.
Materials and Methods
A randomized controlled trial was conducted in the Department of Biochemistry, AIIMS, Bhubaneswar, Odisha, India, during Apr 2017 to Feb 2018. Seventy five uncomplicated type-2 diabetes mellitus patients were enrolled. Group A patients were supplemented with 1 gm of Momordica charantia tablets with oral anti-diabetic agents and Group B with 1.5 gm of Momordica charantia tablets along with oral anti diabetic agents, daily for eight weeks. Group C were treated with oral anti diabetic agents only (Control group). Fasting plasma glucose, Post prandial blood sugar, HbA1C, insulin resistance, lipid profile and Malondialdehyde (MDA) levels were compared between baseline and eight weeks Post supplementation.
Results
Control group had reduced blood sugar levels but it was not significant. A 1 gm of Momordica charantia along with oral anti-diabetic agents had significantly reduced blood sugar, HbA1C, Total cholesterol, LDLc in group A, without improving insulin sensitivity and oxidative stress (MDA). A 1.5 gm of Momordica charantia add on treatment along with stable dose of oral anti-diabetic improved glycaemic profile along with insulin resistance (p<0.05) in type-2 diabetes patients. It reduced total cholesterol, LDLc and increased HDLc levels. It significantly decreased the MDA levels. However, Momordica charantia had no significant effect on Triglycerides and TAG/HDLc ratio in type-2 diabetes mellitus patients.
Conclusion
Add on treatment with 1.5 gm/day of Momordica charantia is effective in glycaemic control, lowering Total cholesterol and oxidative stress. It improves HDLc and insulin resistance in type-2 diabetes mellitus patients.
Introduction
Diabetes mellitus is a complex metabolic condition characterised by hyperglycaemia, insulin resistance being the central mechanism. It is a global health concern, with the projected rise in prevalence from 171 million in 2000 to 366 million in 2010 [1]. Studies have found that one third of patients with diabetes mellitus use some form of complementary and alternative medicine [2,3] that involves use of plant products (herbal products) and dietary supplements as an add on or alternative to mainstream medical treatment. Since ancient times many derivatives of medicinal plants, minerals and organic matter were used to combat diabetes mellitus. The World Health Organization (WHO) has listed 21000 plants out of which 150 species are commercially used for medicinal purpose across the world [3]. Most of the pharmaceuticals commonly used today are derived from the herbal products. Fenugreek (Trigonella foenumgraecum), Gurmar (Gymnema sylvestre), Ivy gourd (Coccinia grandis), Nopal (Opuntia spp.) Ginseng and Momordica charantia (Bitter melon) are such plant products. Recent trend for treating type 2 diabetes is the use of Phytochemicals (Herbal products) with the central theme of “Food as medicine and not medicine as food” [3].
Momordica charantia (Bitter melon, Karela) is well known for treating Diabetes mellitus among Asian Population. Various extracts/components of Momordica charantia are believed to exert their hypoglycaemic effects via different physiological, pharmacological and biochemical modes [4,5]. Earlier studies on animal models have registered that hypoglycaemic effect of Momordica charantia was comparable to that of oral antidiabetic agents like Tolbutamide & Glabenclamide [6,7]. However, the clinical trials in type-2 diabetes mellitus are very few and are with contradictory results. Few case series were published based on supplementation of Fresh Bitter melon (Momordica charantia) fruit juice with dried powder, to type-2 Diabetes mellitus patients for a period of 6-8 weeks. Fasting Plasma glucose level, Glycosuria, Oral glucose tolorence and Hb A1C were measured as the outcome variables with non significant changes [8-10]. A multicentric, randomized double blind trial showed significant change in Fructosamine levels following four weeks of treatment with Momordica charantia tablets [11]. Whereas, few studies have found no significant change in HbA1C levels following three months of treatment with Momordica charantia in type 2 diabetes mellitus patients [12,13]. The clinical trials conducted so far, lack proper control selection, baseline characterization and were with less sample size. Recent study has shown the lipid lowering effect of Momordica charantia but the results were not significant [14]. Studies are now revealing antioxidant property of Momordica charantia in animal models but there is no published data on clinical trial. So this study was conducted to investigate the effect of Momordica charantia on glycaemic profile, insulin resistance, lipid profile, oxidative stress and BMI in type 2 diabetes mellitus patients.
Materials and Methods
This was a Parallel Randomized Controlled trial with participants randomised into three parallel groups with equal randomisation i.e., 1:1, undertaken in 2 phases i.e., baseline and post supplementation phase. The study was conducted in the Department of Biochemistry, All India Institute of Medical Sciences (AIIMS) Bhubaneswar during Apr 2017 to Feb 2018. The research plan was duly approved by the Institute Ethics Committee (IEC) before the commencement of the study. A written informed consent was obtained from all the study participants before any study related intervention. The patients attending the OPD of Endocrinology department were screened for Diabetes Mellitus (DM) as per the WHO diabetes diagnosis criteria [15]. Seventy five Uncomplicated Type 2 DM patients within the age group of 40 to 60 years, under stable dose of individualized oral anti-diabetic agents over a period of four weeks, were enrolled in this study. The dosage and combination of the oral anti-diabetic agents (Metformin and Glibenclamide) based on previous four weeks glycaemic status, were individualized and this treatment modality was maintained throughout the study period. The participants had FBS less than 200 mg/dL and two hour PPBS less than 300 mg/dL with HbA1c less than 8%. Type-2 Diabetes Mellitus patients with microvascular or macrovascular complication as well as type-1 Diabetes mellitus patients were excluded from this study. Patients with hepatic failure, past history of Acute Myocardial Infarction, under lipid lowering drugs, antioxidants or history of any other herbal product intake were not enrolled in the present study. The sample size was calculated using the standard formula with r=1 i.e., ratio of control to case, σ=Standard deviation of the outcome variables=5, Zβ=1.65 for 95% power, Zα=1.96 for 0.05 significance level, Difference=half of standard deviation=2.5 with 5% attrition. Participants satisfying the inclusion and exclusion criteria, willing to participate in this study were advised to abstain from alcohol, smoking, and heavy carbohydrate diet for seven days prior to the test, till the completion of the study period, which was assessed by one to one counselling before enrolling them in this study. The Study participants were instructed to report in the biochemistry laboratory following 12 hours of overnight fast, provided with the consent form and after obtaining the informed written consent, 5 mL of fasting venous blood sample was drawn to carry out the biochemical analysis. The participants were advised to take routine meal along with the prescribed oral anti-diabetic agents and report to the laboratory two hours after the food, to provide plasma sample for 2 hours Post Prandial Blood sugar (PPBS) estimation. Fasting Plasma Glucose (FPG), 2 hours Post Prandial Blood sugar (PPBS) Total Cholesterol (TC), High Density Lipoprotein (HDLc), Low Density Lipoprotein (LDLc), Triglycerides (TAG), HbA1C were estimated by Fully automated Chemistry Analyser (Beckman Coulter, AU-5800) using system compatible reagent packs. The fasting Serum Insulin level was estimated by Enzyme Linked Immuno Sorbent Assay (ELISA) kits based on quantitative competitive enzyme immunoassay technique. Insulin resistance was assessed using HOMA-IR calculation [16]. To analyse the oxidative state, serum Malondialdehyde (MDA) levels were estimated by colorimetric method. General health characteristics, height, weight were measured to find out BMI. Resting Blood Pressure was measured twice and the mean value was recorded. All these data were registered as the baseline value.
Random numbers were generated using Excel sheet and a block of first 25 random numbers (participant Code) were allocated in group A, next 25 numbers were allocated in group B, rest 25 numbers were in group C. Patients were given participant code numbers sequentially depending on the order of their enrollment in this study. The randomisation was done by Principal Investigator. The participants did not know in advance which treatment they will get. This was a single blinded RCT. A total of 25 subjects included in group A satisfying the selection criteria were informed about the trial they will be undergoing and supplemented with stable dose of oral anti-diabetic agents and 1 gm of commercially available Momordica charantia tablets daily for a period of eight weeks. A total of 25 subjects in group B were supplemented with stable dose of oral anti-diabetic agents (Individualised dose of combination of the Metformin and Glibenclamide based on previous four weeks glycaemic status) and 1.5 gm Momordica charantia tablets everyday for eight weeks. Rest 25 subjects were treated as control (group C) with stable dose of oral anti-diabetic agents and placebo i.e., Riboflavin capsules. All patients followed the WHO guidelines for Total Calorie requirement with a range of 1600-2200 Calories per Day, Physical activity of Brisk walking 30-45 min/day, 5 days in a week as per ADA guidelines [17].
Following eight weeks of Momordica charantia supplementation, second round of investigations with fasting plasma glucose, HbA1c, insulin, lipid profile and MDA were conducted along with BMI and Blood Pressure measurement in all the three groups [Table/Fig-1]. These data were recorded as the post supplementation values.
Statistical Analysis
A comparative statistical analysis was done between the post supplementation values and the baseline values using SPSS Version 21. The values of continuous variables were expressed as mean±SD. Differences in variables between the post supplementation values and the baseline values were compared by paired t-test. A p-value <0.05 was considered as statistically significant.
Results
Seventy five uncomplicated type-2 diabetes mellitus patients were enrolled in this study, attending the OPD of Endocrinology and metabolism department of AIIMS, Bhubaneswar. Most of the participants were elderly people with male predominance residing in coastal region of Odisha. During the entire study period, only two participants reported mild gastrointestinal discomfort in the form of transient dyspepsia and diarrhoea that abated after 5-7 days of probiotics and oral rehydration therapy and both of them finished the study successfully. Compliance, assessed by counting the number of tablets returned on each visit, was found satisfactory. One patient stopped intake of oral anti-diabetic drug for two days but again restarted immediately after it. There were no dropouts during the entire study period.
The baseline characteristics [Table/Fig-2] showed homogenous distribution of study participants among the three groups i.e., Group A, Group B and Group C. There was no significant difference in BMI, blood pressure, glycaemic profile and lipid profile.
Baseline characteristics of the study population.
| S. No. | Parameters | Group A | Group B | Group C |
|---|
| 1 | BMI (kg/m2) | 26.3±3.7 | 27.95±3.2 | 28.9±5.4 |
| 2 | SBP (mm of Hg) | 136.4±15.7 | 131.3±8.6 | 140.3±10.8 |
| 3 | DBP (mm of Hg) | 89.6±12.1 | 84.3±5.9 | 89.8±7.7 |
| 4 | FPG (mg/dL) | 158.4±19.9 | 144.5±18.2 | 155.9±12.3 |
| 5 | PPBS (mg/dL) | 218.9±23.2 | 228.9±19.9 | 221.4±23.8 |
| 6 | HbA1C (%) | 7.6±0.7 | 7.3±0.47 | 7.1±0.49 |
| 7 | Insulin (mIU/L) | 40.2±4.5 | 43.5±5.8 | 44.8±8.1 |
| 8 | Total cholesterol (mg/dL) | 207.3±18.3 | 192.6±15.6 | 188.5±18.1 |
| 9 | Triglycerides (mg/dL) | 135.2±35.9 | 172.3±17.5 | 133.5±23.9 |
| 10 | LDLc (mg/dL) | 122.8±21.4 | 118.1±13.9 | 113.8±16.9 |
| 11 | HDLc (mg/dL) | 44.2±6.6 | 40.1±3.9 | 45.6±5.1 |
| 12 | MDA (nmol/mL) | 2.12±0.2 | 2.1±0.2 | 2. 0±0.3 |
| 13 | TAG/HDL | 3±0.87 | 4.4±1.6 | 2.9±0.73 |
| 14 | LDL/HDL | 2.85±0.48 | 2.7±0.6 | 2.7±0.48 |
| 15 | Insulin Resistance | 12.78±2.18 | 13.5±1.5 | 14.1±1.28 |
Baseline characteristics were compared and no significant difference was observed.
Primary outcome with stable dose of oral anti-diabetic agent in control group i.e., in group C [Table/Fig-3] showed a fall in Fasting Plasma Glucose (FPG) and 2 hours Post Postprandial Blood sugar (PPBS) but it was not statistically significant. There was no significant change in HbA1C, lipid profile, Insulin levels and MDA suggesting that the study participants were non-responsive to the stable dose of oral anti-diabetic agents. This also rules out the confounding effect of oral anti-diabetic agents on lipid profile and oxidative status.
Primary outcome in control group (Group C) following oral anti diabetic agent.
| S. No. | Parameters | Pre-supplementation | Post supplementation |
|---|
| 1 | BMI (kg/m2) | 28.9±5.4 | 28.55±5.0 |
| 2 | SBP (mm of Hg) | 140.3±10.8 | 139.50±9.28 |
| 3 | DBP (mm of Hg) | 89.8±7.7 | 89.80±7.97 |
| 4 | FPG (mg/dL) | 155.9±12.3 | 151.60±16.18 |
| 5 | PPBS (mg/dL) | 221.4±23.8 | 219.95±25.65 |
| 6 | HbA1C (%) | 7.1±0.49 | 7.15±0.47 |
| 7 | Insulin (mIU/L) | 44.8±8.1 | 45.75±6.65 |
| 8 | Total cholesterol (mg/dL) | 188.5±18.1 | 195.90±31.68 |
| 9 | Triglycerides (mg/dL) | 133.5±23.9 | 134.45±31.92 |
| 10 | LDLc (mg/dL) | 113.8±16.9 | 114.80±12.60 |
| 11 | HDLc (mg/dL) | 45.6±5.1 | 46.35±5.67 |
| 12 | MDA (nmol/mL) | 2. 0±0.3 | 2.2±0.61 |
| 13 | TAG/HDL | 2.9±0.73 | 2.89±0.38 |
| 14 | LDL/HDL | 2.7±0.48 | 2.74±0.42 |
| 15 | Insulin Resistance | 14.1±1.28 | 14.64±1.04 |
Add on treatment with 1 gm of Momordica charantia [Table/Fig-4] for eight weeks in group A participants showed significant fall in systolic blood pressure. BMI was not markedly affected. There was improvement in glycaemic profile i.e., significant fall in FPG and PPBS. However, the serum insulin levels and Insulin resistance were not significantly changed. A reduction(p<0.05) in Total Cholesterol (Total cholesterol) after eight weeks of 1 gm Momordica charantia treatment as compared to baseline was observed. This was associated with fall in LDLc and LDLc/HDLc ratio (p<0.05). HDLc was found increased suggesting the favourable effect of Momordica charantia on lipid profile in type-2 diabetes mellitus patients. No significant change was observed following 1 gm of Momordica charantia treatment on Oxidative stress (MDA).
Effect of add-on treatment with 1 gm momordica charantia (Group A).
| S. No. | Parameters | Pre-supplementation (Baseline) | Post supplementation (After 8 weeks) | p-values |
|---|
| 1 | BMI (kg/m2) | 26.3±3.7 | 24.30±3.75 | 0.151 |
| 2 | SBP (mm of Hg) | 136.4±15.7 | *131.10±13.03 | 0.041 |
| 3 | DBP (mm of Hg) | 89.6±12.1 | *86.40±10.77 | 0.043 |
| 4 | FPG (mg/dL) | 158.4±19.9 | *142.70±9.8 | 0.035 |
| 5 | PPBS (mg/dL) | 218.9±23.2 | *196.15±15.45 | 0.027 |
| 6 | HbA1C (%) | 7.6±0.7 | 7.42±0.5 | 0.111 |
| 7 | Insulin (mIU/L) | 40.2±4.5 | 36.45±3.74 | 0.173 |
| 8 | Total cholesterol (mg/dL) | 207.3±18.3 | *188.50±19.08 | 0.018 |
| 9 | Triglycerides (mg/dL) | 135.2±35.9 | 133.50±13.92 | 0.142 |
| 10 | LDLc (mg/dL) | 122.8±21.4 | *113.85±16.90 | 0.017 |
| 11 | HDLc (mg/dL) | 44.2±6.6 | *45.60±5.19 | 0.049 |
| 12 | MDA (nmol/mL) | 2.12±0.2 | 2.03±0.13 | 0.660 |
| 13 | TAG/HDL | 3±0.87 | 2.95±0.73 | 1.000 |
| 14 | LDL/HDL | 2.85±0.48 | *2.73±0.48 | 0.042 |
| 15 | Insulin Resistance | 12.78±2.18 | 11.94±1.94 | 0.682 |
*Indicates p<0.05 (Significant difference as compared to respective baseline levels) by student’s t-test
Add on treatment with 1.5 gm of Momordica charantia in group B [Table/Fig-5] patients showed significant improvement (p<0.05) in systolic and diastolic blood pressure. A fall in body mass index with 1.5 gm Momordica charantia (p<0.05) was observed.
Effect of momordica charantia (1.5 gm) on body mass index and blood pressure.
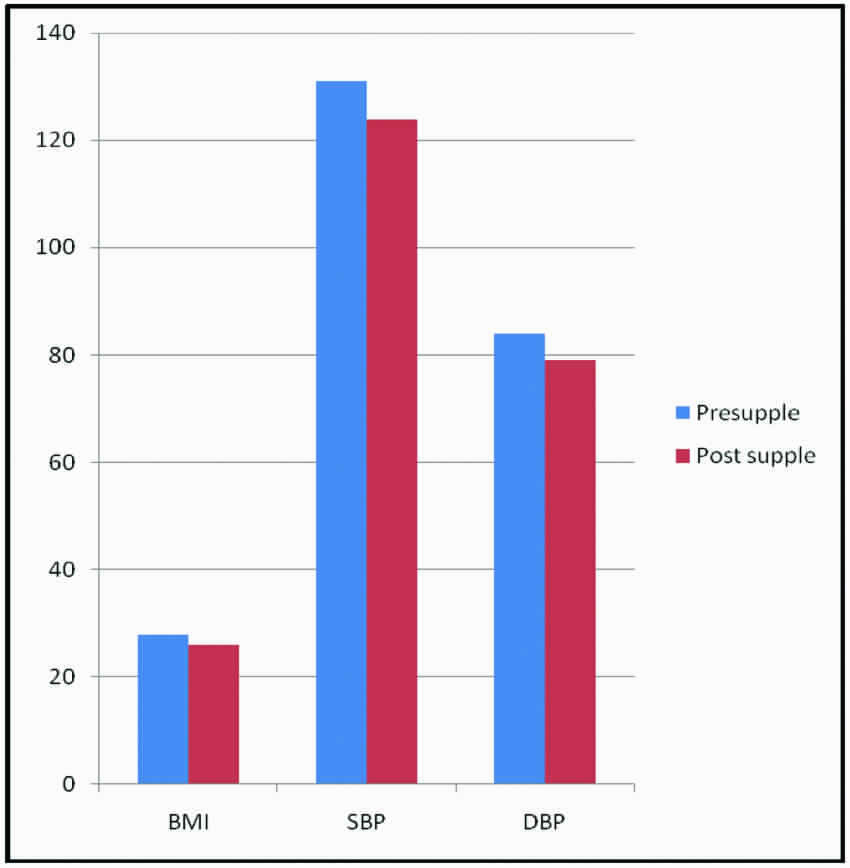
[Table/Fig-6] showed the effect of 1.5 gm of Momordica charantia on glycaemic profile, Lipid profile and MDA levels. FPG, PPBS and HbA1C reduced significantly. There was a significant change in the Insulin levels associated with improvement in Insulin resistance suggesting the beneficial effect of 1.5 gm Momordica charantia in β-cell function and Insulin sensitivity. Though the Total Cholesterol, LDLc levels and LDL/HDL ratio reduced (p<0.05), there was no significant effect on Triglycerides level and TG/HDL ratio, following 1.5 gm Momordica charantia add on treatment. Serum HDL level increased significantly. Dyslipidemia being a widely accepted independent risk factor for coronary heart disease, Momordica charantia in a dose of 1.5 gm effectively improves lipid metabolism thereby reduced the cardiovascular risk in type 2 diabetes mellitus patients. A significant reduction in the MDA levels pointing the antioxidant role of Momordica charantia in present study participants [Table/Fig-6] was found.
Effect of Momordica charantia (1.5 gm) on glycaemic profile, lipid profile and oxidative state in Group-B.
| S. No. | Parameters | Pre-supplementation (Baseline) | Post supplementation (after 8 weeks) | p-value |
|---|
| 1 | FPG (mg/dL) | 144.5±18.2 | *102.82±11.16 | 0.021 |
| 2 | PPBS (mg/dL) | 228.9±19.9 | *167.38±16.11 | 0.009 |
| 3 | HbA1C (%) | 7.3±0.47 | *6.2±1.26 | 0.049 |
| 4 | Insulin (mIU/L) | 43.5±5.8 | *29.40±5.22 | 0.018 |
| 5 | Total cholesterol (mg/dL) | 192.6±15.6 | *164.86±12.24 | 0.041 |
| 6 | Triglycerides (mg/dL) | 172.3±17.5 | 172.30±19.68 | 0.982 |
| 7 | LDLc (mg/dL) | 118.1±13.9 | *90.95±17.27 | 0.039 |
| 8 | HDLc (mg/dL) | 40.1±3.9 | *46.95±4.83 | 0.043 |
| 9 | MDA (nmol/mL) | 2.1±0.2 | *1.78±0.20 | 0.038 |
| 10 | TAG/HDL | 4.4±1.6 | 4.4±1.56 | 0.826 |
| 11 | LDL/HDL | 2.7±0.6 | *2.58±0.59 | 0.041 |
| 12 | Insulin Resistance | 13.5±1.5 | *9.66±1.08 | 0.027 |
*Indicates p<0.05 (significant difference as compared to respective baseline levels following 1.5 gm of M. charantia supplementation) by student’s t-test
Discussion
Type 2 diabetes mellitus patients have a substantial risk of developing microvascular and macrovascular complications. This risk is independent to the degree of glycaemic control [18]. So glycaemic control alone is not associated with any reduction in risk of developing complications. Thus, treatment intended to improve glycaemic control would be beneficial in long term, if it would have a favourable effect on lipid profile as well as oxidative stress.
Add on supplementation with herbal products along with the conventional therapy have shown such therapeutic potential. Momordica charantia (bitter melon) is a popular fruit used as a supplementary agent to treat Diabetes Mellitus. The proposed hypoglycaemic effect of bitter melon could be attributed to its role in insulin secretion and insulin resistance [19]. The results of this study indicate that Momordica charantia in a dose of 1.5 gm is more effective in controlling glycaemic profile. Hafizur RM et al., suggested similar beneficial effect of Momordica in blood sugar levels and they concluded that such effect could be attributed to the ability of Momordica charantia to maintain the structural integrity of pancreatic islets and release of hormones [20]. The hypoglycaemic effect of Momordica charantia could be due to the active components like Charantin, Vicine and Polypeptide that are known to have structural similarity with human insulin [21]. The Insulino-mimetic action of these active components enhances peripheral uptake of glucose by skeletal muscles and adipose tissue, reducing the blood glucose levels [22]. They prevent intestinal glucose uptake resulting in improvement in glucose tolerance [23]. Charatin is known to suppress key enzymes of gluconeogenesis, activates enzymes of HMP shunt and glycogenesis, thus favouring a good glycaemic control [24]. β-cell dysfunction and insulin resistance are the two central mechanisms of type-2 diabetes mellitus. Earlier studies have documented that Momordica charantia can stimulate insulin secretion from the pancreatic β cells [25,26]. It supports restoration of the functional pancreatic β cells that secrete Insulin (Insulin Secretagogue like effect) thereby improving β cell function. Abundant biochemical data have shed light upon the possible mechanisms of Momordica charantia facilitating the AMP activated protein kinase system i.e., post insulin receptor signalling cascade, thus improving insulin resistance [26].
The lipid lowering effect of Momordica charantia has been extensively studied in animal models. The clinical trials are few with contradictory findings [27,28]. Kasbia GS et al., reported non significant effect of freeze dried extract of Momordica (50 mg/kg) in non-diabetic overweight men [29]. However, the study conducted by Rahman IU et al., showed significant hypolipidemic effect in diabetes but the hypoglycaemic effect was found weaker as compared to glibenclamide [25]. Momordica charantia is found effective in lowering the total cholesterol levels in the present study but no beneficial effect is observed on triglycerides. The lipid lowering effect of Momordica charantia is multifactorial. It decreases body weight by increasing the fatty acid oxidation facilitating the CPT-1 and CPT-2 Carnitine activity in the inner mitochondrial matrix [12]. Extract of Momordica charantia modulate fat mobilizing kinases i.e., AMP kinase and transcription factors like PPARα and PPARγ in the liver and skeletal muscle, thereby affecting adipocyte differentiation and preventing adipocyte hypertrophy [30]. Experiments on animal models have found decreased cholesterol with normalised leptin and insulin concentration with Momordica charantia supplementation [23,31]. Recent clinical trials have documented decreased fructosamine along with decreased incidence of metabolic syndrome with different doses of bitter melon [32-34]. The decrease in BMI and blood pressure in the present study goes well with these earlier findings pointing towards its favourable effect on fat metabolism. Momordica charantia by improving dyslipidemia and BMI could prevent cardiovascular risk in type 2 diabetes mellitus.
Hyperglycaemia prevailing in diabetes mellitus induces glycation of proteins. These glycated proteins rearrange to form Amadori products that in presence of transition metals and oxygen undergo autoxidation to form free radicals. Significant fall in MDA levels was observed with 1.5 gm Momordica charantia add on treatment in the present study. Horax R et al., reported similar antioxidant activity of Momordica [35]. The free radical scavenging role of Momordica is also documented by a clinical trial conducted by Wu SJ et al., [36]. From this study they have concluded that the presence of Polyphenols, flavonoids and flavonols in Momordica could have free radical scavenging activity. Momordicacharantia contains numerous amino acids that might reduce Advanced Glycated End product (AGE) formation [37]. Accumulation of cross linked AGEs in tissues is believed to be one factor responsible for the long-term complications of diabetes mellitus. A significantly increased levels of antioxidant enzymes i.e., glutathione peroxidase, Catalase, Superoxide dismutase and Reduced Glutathione levels were found in sucrose fed rat model, following Momordica charantia therapy [38]. Momordica charantia reduce the process of lipid peroxidation there by preventing the glycation of proteins which could delay the onset of complications in type 2 diabetes mellitus cases.
Limitation
This study could have better impact if it had larger sample size. Inclusion of patients with impaired glucose tolerance and non diabetic control group, would better evaluate the therapeutic potential of Momordica charantia on derangement of carbohydrate metabolism in diabetes mellitus patients.
Conclusion
Dyslipidemia and oxidative stress are the common association in diabetes mellitus. Conventional treatment with oral anti-diabetic agents control blood sugar without any changes in Insulin metabolism. Add on treatment with Momordica Charantia is more effective in reducing blood sugar and HbA1C levels. It reduces total cholesterol and LDLc, improves HDLc levels showing the cardio-protective effect. It has a beneficial role on oxidative stress and improves insulin resistance in type 2 diabetes mellitus patients.
*Indicates p<0.05 (Significant difference as compared to respective baseline levels) by student’s t-test
*Indicates p<0.05 (significant difference as compared to respective baseline levels following 1.5 gm of M. charantia supplementation) by student’s t-test
[1]. Shaw JE, Sicree RA, Zimmet PZ, Global estimates of the prevalence of diabetes for 2010 and 2030Diabetes Res Clin Pract 2010 87:04-14.10.1016/j.diabres.2009.10.00719896746 [Google Scholar] [CrossRef] [PubMed]
[2]. Joseph B, Jini D, Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potencyAsian Pacific Journal of Tropical Disease 2013 3(2):93-102.10.1016/S2222-1808(13)60052-3 [Google Scholar] [CrossRef]
[3]. Singh U, Singh S, Kochhar A, Therapeutic potential of antidiabetic neutraceuticalsPhytopharmacol 2012 2(1):144-69. [Google Scholar]
[4]. Leung L, Birtwhistle R, Kotecha J, Hannah S, Cuthbertson S, Anti-diabetic and hypoglycaemic effects of Momordica charantia (bitter melon): a mini reviewBr J Nutr 2009 102:1703-08.10.1017/S000711450999205419825210 [Google Scholar] [CrossRef] [PubMed]
[5]. Bhushan MS, Rao CHV, Ojha SK, Vijayakumar M, Verma A, An analytical review of plants for anti diabetic activity with their phytoconstituent and mechanism of actionIJPSR 2010 1(1):29-46. [Google Scholar]
[6]. Raman BV, Krishna NV, Rao NB, Saradhi PM, Rao BMV, Plants with antidiabetic activities and their medicinal valuesInt Res J Pharm 2012 3(3):11-15. [Google Scholar]
[7]. Hameed M, Al-Bahrani A, The role of momordica charantia in reducing the level of glucose in miceInt J Curr Microbiol App Sci 2016 5(8):470-78.10.20546/ijcmas.2016.508.050 [Google Scholar] [CrossRef]
[8]. Akhtar MS, Trial of Momordica charantia Linn (Karela) powder in patients with maturity-onset diabetesJ Pakistan Med Assoc 1982 32:106-07. [Google Scholar]
[9]. Leatherdale BA, Panesar RK, Singh G, Atkins TW, Bailey CJ, Bignell AH, Improvement in glucose tolerance due to Momordica charantia (karela)Br Med J 1981 282:1823-24.10.1136/bmj.282.6279.18236786635 [Google Scholar] [CrossRef] [PubMed]
[10]. Khanna P, Jain SC, Panagariya A, Dixt VP, Hypoglycaemic activity of polypeptide-p from a plant sourceJ Nat Prod 1981 44:648-55.10.1021/np50018a0027334382 [Google Scholar] [CrossRef] [PubMed]
[11]. Wehash FE, Abpo-Ghanema II, Saleh RM, Some physiological effects of Momordica charantia and Trigonella foenum-graecum extracts in diabetic rats as compared with cidophageWorld Academy of Science, Engineering and Technology 2012 64:1206-14. [Google Scholar]
[12]. Dans AM, Villarruz MV, Jimeno CA, Anthony M, Javelosab U, Chuaa J, The effect of Momordica charantia capsule preparation on glycaemic control in type 2 diabetes mellitus needs further studiesJ Clin Epidemiol 2007 60:554-59.10.1016/j.jclinepi.2006.07.00917493509 [Google Scholar] [CrossRef] [PubMed]
[13]. Tsi C, Chen EC, Tsay H, Huang C, Wild bitter gourd improves metabolic syndrome: A preliminary dietary supplementation trialNutr J 2012 11:04-07.10.1186/1475-2891-11-422243626 [Google Scholar] [CrossRef] [PubMed]
[14]. Rahman IU, Basir M, Salman M, Idrees M, Khan MI, Bitter melon (Momordica charantia) reduces serum sialic acid in type 2 diabetics: Evidence to delay the process of atherosclerosisChin Med 2011 2:125-29.10.4236/cm.2011.24021 [Google Scholar] [CrossRef]
[15]. Goedegebure EAR, Koning SH, Hoogenberg K, Korteweg FJ, Lutgers HL, Diekman MJM, Pregnancy outcomes in women with gestational diabetes mellitus diagnosed according to the WHO-2013 and WHO-1999 diagnostic criteria: a multicentre retrospective cohort studyBMC Pregnancy Childbirth 2018 18(1):15210.1186/s12884-018-1810-529747601 [Google Scholar] [CrossRef] [PubMed]
[16]. Singh Y, Garg MK, Tandon N, Marwaha RK, A study of insulin resistance by HOMA-IR and its Cut-off value to identify metabolic syndrome in urban Indian adolescentsJ Clin Res Pediatr Endocrinol 2013 5(4):245-51.10.4274/Jcrpe.112724379034 [Google Scholar] [CrossRef] [PubMed]
[17]. Chamberlain JJ, Johnson EL, Leal S, Rhinehart AS, Shubrook JH, Peterson L, Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018Ann Intern Med 2018 168(9):640-50.10.7326/M18-022229610837 [Google Scholar] [CrossRef] [PubMed]
[18]. Yin RV, Lee NC, Hirpara H, OJ Phung, The effect of bitter melon (Mormordica charantia) in patients with diabetes mellitus: a systematic review and meta-analysis CitationNutrition & Diabetes 2014 4:e14510.1038/nutd.2014.4225504465 [Google Scholar] [CrossRef] [PubMed]
[19]. Fuangchan A, Sonthisombat P, Seubnukarn T, Chanouan R, Chotchaisuwat P, Sirigulsatien V, Hypoglycaemic effect of bitter melon compared with metformin in newly diagnosed type 2 diabetes patientsJ Ethnopharm 2017 134:422-28.10.1016/j.jep.2010.12.04521211558 [Google Scholar] [CrossRef] [PubMed]
[20]. Hafizur RM, Kabir N, Chishti S, Modulation of pancreatic β-cells in neonatally streptozotocin-induced type 2 diabetic rats by the ethanolic extract of Momordica charantia fruit pulpNatural Product Research 2011 25(4):353-67.10.1080/1478641100376690421328131 [Google Scholar] [CrossRef] [PubMed]
[21]. Patel DK, Prasad SK, Kumar R, Hemelatha S, An overview on antidiabetic medicinal plants having insulin mimetic propertyAsian Pac J Trop Biomed 2012 2:320-30.10.1016/S2221-1691(12)60032-X [Google Scholar] [CrossRef]
[22]. Sarandan H, Botau D, Ianculov I, Radu F, Rada O, Morar D, The hypoglicaemic effect of Momordica charantia Linn in normal and alloxan induced diabetic rabbitsScientific Papers: Animal Science and Biotechnologies 2010 43(1):516-18. [Google Scholar]
[23]. Chun CS, Cheng HL, Wei LL, Effect of momordica Charantia on Insulin resistance and visceral obesity in mice on high fat dietDiabetes Research and Clinical Practice 2008 81:134-43.10.1016/j.diabres.2008.04.02318550200 [Google Scholar] [CrossRef] [PubMed]
[24]. Wilai T, Uthai S, Pariya P, Supatra P, Umaporn U, Surat K, Pilot study: hypoglycaemic and antiglycation activities of bitter melon (Momordica charantia L.) in type 2 diabetic patientsJournal of Pharmacy Research 2013 6(8):859-64.10.1016/j.jopr.2013.08.007 [Google Scholar] [CrossRef]
[25]. Rahman IU, Khan RU, Rahman KU, Bashir M, Lower hypoglycaemic but higher antiatherogenic effects of bitter melon than glibenclamide in type 2 diabetic patientsNutr J 2015 14:1310.1186/1475-2891-14-1325623883 [Google Scholar] [CrossRef] [PubMed]
[26]. Singh J, Cumming E, Manoharan G, Kalasz H, Adeghate E, Medicinal chemistry of the anti-diabetic effects of Momordica Charantia: active constituents and modes of actionsOpen Med Chem J 2011 5:70-77.10.2174/187410450110501007021966327 [Google Scholar] [CrossRef] [PubMed]
[27]. Abdollah M, Zuki ABZ, Goh YM, Rezaeizadeh A, Noordin MM, The effects of Momordica charantia on the liver in streptozotocin induced diabetes in neonatal ratsAfr J Biotechnol 2010 9(31):5004-12. [Google Scholar]
[28]. Cheng HL, Huang HK, Chang CI, Tsai CP, Chou CH, A cell-based screening identifies compounds from the stem of Momordica charantia that overcome insulin resistance and activate AMP activated protein kinaseJ Agric Food Chem 2008 27:6835-43.10.1021/jf800801k18656931 [Google Scholar] [CrossRef] [PubMed]
[29]. Kasbia GS, Arnason JT, Imbeault P, No effect of acute, single dose oral administration of Momordica charantia Linn., on glycaemia, energy expenditure and appetite: a pilot study in non-diabetic overweight menJ Ethnopharmacol 2009 126(1):127-33.10.1016/j.jep.2009.07.03519665538 [Google Scholar] [CrossRef] [PubMed]
[30]. Huang HL, Hong YW, Wong YH, Chen YN, Chyuan JH, Huang CJ, Bitter melon (Momordica charantia L.) inhibits adipocyte hypertrophy and down regulates lipogenic gene expression in adipose tissue of diet-induced obese ratsBr J Nutr 2008 99:230-39.10.1017/S000711450779394717651527 [Google Scholar] [CrossRef] [PubMed]
[31]. Tsai CH, Chen EC, Tsay HS, Huang CJ, Wild bitter gourd improves metabolic syndrome: a preliminary dietary supplementation trialNutr J 2012 11(1):410.1186/1475-2891-11-422243626 [Google Scholar] [CrossRef] [PubMed]
[32]. Yu Y, Zhang XH, Ebersole B, Ribnicky D, Wang ZQ, Bitter melon extract attenuating hepatic steatosis may be mediated by FGF21 and AMPK/Sirt1 signaling in miceSci Rep 2013 5(3):314210.1038/srep0314224189525 [Google Scholar] [CrossRef] [PubMed]
[33]. Shih CC, Shlau MT, Lin CH, Wu JB, Momordica charantia ameliorates insulin resistance and dyslipidemia with altered hepatic glucose production and fatty acid synthesis and AMPK phosphorylation in high-fat-fed micePhytother Res 2014 28(3):363-71.10.1002/ptr.500323610006 [Google Scholar] [CrossRef] [PubMed]
[34]. Matsui S, Yamane T, Takita T, Oishi Y, Kobayashi-Hattori K, The hypocholesterolemic activity of Momordica charantia fruit is mediated by the altered cholesterol- and bile acid regulating gene expression in rat liverNutr Res 2013 33(7):580-85.10.1016/j.nutres.2013.05.00223827133 [Google Scholar] [CrossRef] [PubMed]
[35]. Horax R, Hettiarachchy N, Chen P, Extraction, quantification and antioxidant activities of phenolics from pericarp and seeds of bitter melons (Momordica charantia) harvested at three maturity stages (immature, mature, and ripe)Journal of Agricultural and Food Chemistry 2010 58(7):4428-33.10.1021/jf902957820225855 [Google Scholar] [CrossRef] [PubMed]
[36]. Wu SJ, Ng L-T, Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in TaiwanLWT: Food Science and Technology 2008 41(2):323-30.10.1016/j.lwt.2007.03.003 [Google Scholar] [CrossRef]
[37]. Sahib NG, Hamid AA, Saari N, Abas F, Dek MSP, Rahim M, Anti-pancreatic lipase and antioxidant activity of selected tropical herbsInternational Journal of Food Properties 2012 15(3):569-78.10.1080/10942912.2010.494754 [Google Scholar] [CrossRef]
[38]. Tripathi UN, Chandra D, The plant extracts of Momordica charantia and Trigonella foenum graecum have antioxidant and anti-hyperglycaemic properties for cardiac tissue during diabetes mellitusOxid Med Cell Longev 2009 2(5):290-96.10.4161/oxim.2.5.952920716916 [Google Scholar] [CrossRef] [PubMed]