The incidence of multiple births has been on the rise over the last few decades. This is not only due to the inadvertent use of ovulation induction drugs in assisted reproductive techniques, but also due to the increase in the maternal age [1]. In a study done by Zhang X et al., the prevalence rate of the birth defects was 156.1 per 10000 births (95% CI: 146.3-165.8); 124.6 per 10000 births (95% CI: 111.1-138.1) in urban areas and 179.4 per 10000 births (95% CI: 165.5-193.3) in rural areas (p<0.05) [2]. There is evidence that multiple births have an increased risk of CA relative to singleton births. The excess in CAs has been associated with the splitting of the zygote and with vascular accidents as a result of blood clots or other debris moving across a shared or joined placenta in monozygotic twins [3]. There can be difficulty in management of the pregnancy and delivery if multiple pregnancies co-exist with congenital malformation of one or more fetuses.
Materials and Methods
This was a clinical, non-interventional, hospital-based case control study conducted in S.C.B. Medical College and Hospital, Cuttack, Odisha, over a period of one year. Data were collected from September 2015 to August 2016. Total number of the live births, still births, and abortions >20 weeks were collected. Number of multiple births and singleton deliveries with CAs were recorded. Total number of multiple births with birth defects and equal number of multiple births without defect (consecutive cases of both abnormal and normal multiple births) were included in the study. Details of multiple births such as gestational age, maternal risk factors, Ultrasonographic (USG) findings were recorded. Data including age, parity, previous abortions, infant deaths, socioeconomic status, and habitat, history of fever in 1st trimester, diabetes mellitus, hypertension and any other drug intake in 1st trimester by the mother were collected. Investigations to detect anaemia, folic acid level, hyperlipidemia and diabetes were done. The cut-off levels of various parameters for anaemia, diabetes mellitus and folic acid deficiency were fixed at haemoglobin <10gm/dl, FBS >110 mg/dl, plasma folic acid level <2 ng/mL respectively. The cut-off levels to detect hyperlipidemia were taken as total cholesterol >200 mg/dl, LDL cholesterol >100 mg/dl, triglyceride (TG)>150mg/dl. Detailed external examination was conducted to find out any anomaly and subtype the defect as per international standard classification [5]. In current pregnancy, gestational age at delivery was calculated from Last Menstrual Period (LMP)/early pregnancy USG and birth rate was taken. In this way, 15 cases of twin pregnancies with birth defects were taken. After delivery of each case, next multiple birth without any birth defect was taken as control (15 in number). Similar numbers of normal newborns were enrolled as controls. The relevant data of controls were also taken.
Results
Total births in the above period were 10817, inclusive of 278 multiple births and there were 145 with CA among singletons and 15 among multiple births. Out of these 278 multiple births, there were 5 triplets, and rest were twin births. Among 15 congenital anomalous cases, in six cases both of the babies were affected and in rest 9, one of the babies was affected. Out of a total of 30 fetuses, 16 are live born and 14 were still born/Intrauterine Deaths (IUD). The prevalence of congenital anomaly is 123.35/10,000 births and 539.57/10,000 births in singleton and multiple births respectively. The occurrence of CA in multiple births is 4 to 5 (4.37) times higher than that of singleton births and is statistically significant. Weinberg formula was used for determining proportion of Monozygotic (MZ) and Dizygotic (DZ) twins. Accordingly all Opposite Sex (OS) twins were classified as DZ, where as among Same Sex (SS) twins 50% are considered MZ and 50% DZ. So among total 273 twins in the present study 184 were SS twins and 89 were OS twins. There were five triplets, four SS and one OS. So 184 were DZ twins and 94 were MZ twins [Table/Fig-1]. Zygosity is important as CAs is more frequent among MZ twins.
Details of multiple births with zygosity.
| Zygosity | Sex | Monozygotic | Dizygotic |
|---|
| Twins | Same sex | 184 | 92 | 92 |
| Opposite sex | 89 | 00 | 89 |
| Triplets | Same sex | 04 | 02 | 02 |
| Opposite sex | 01 | 00 | 01 |
| Total | 278 | 94 | 184 |
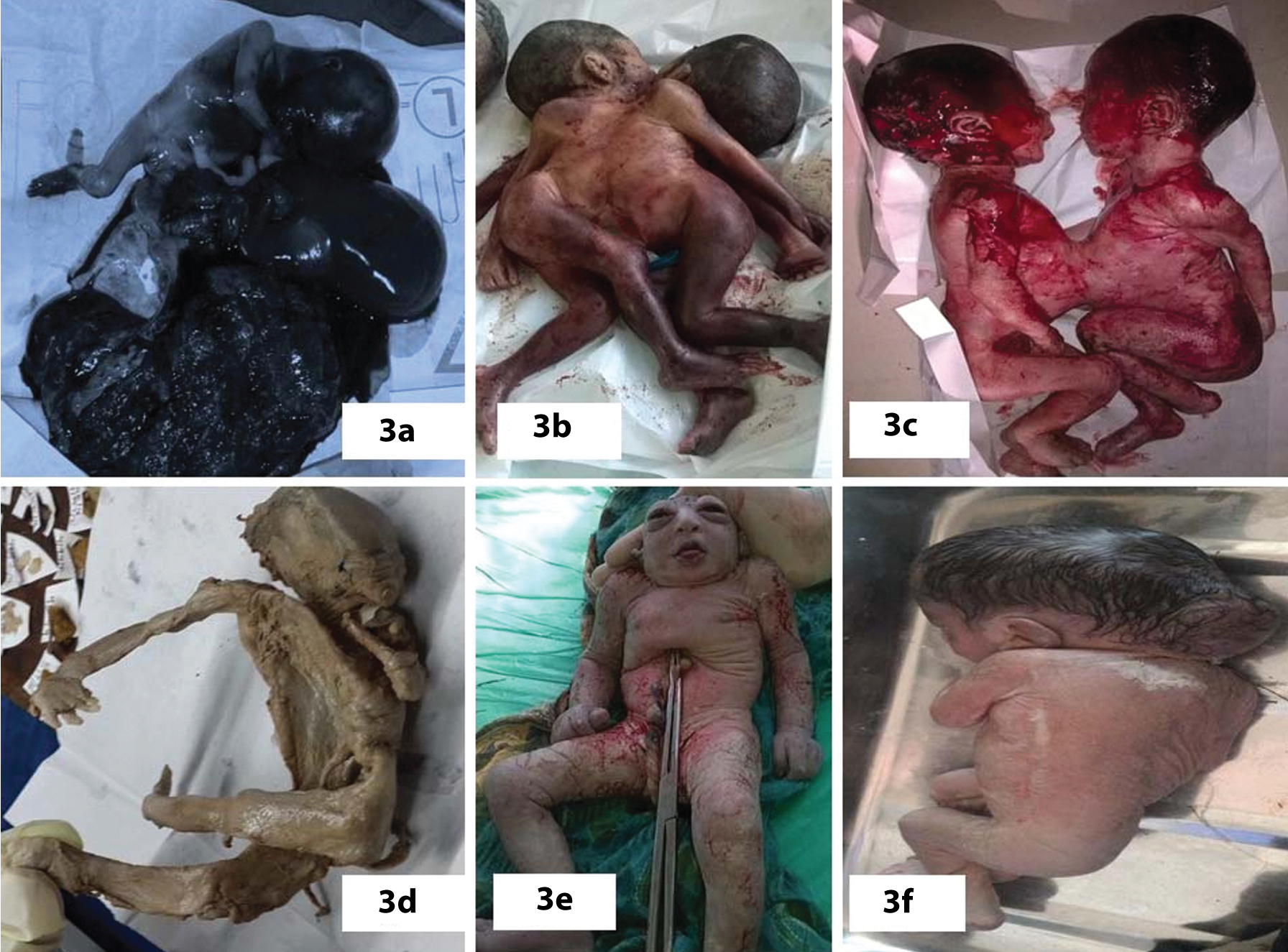
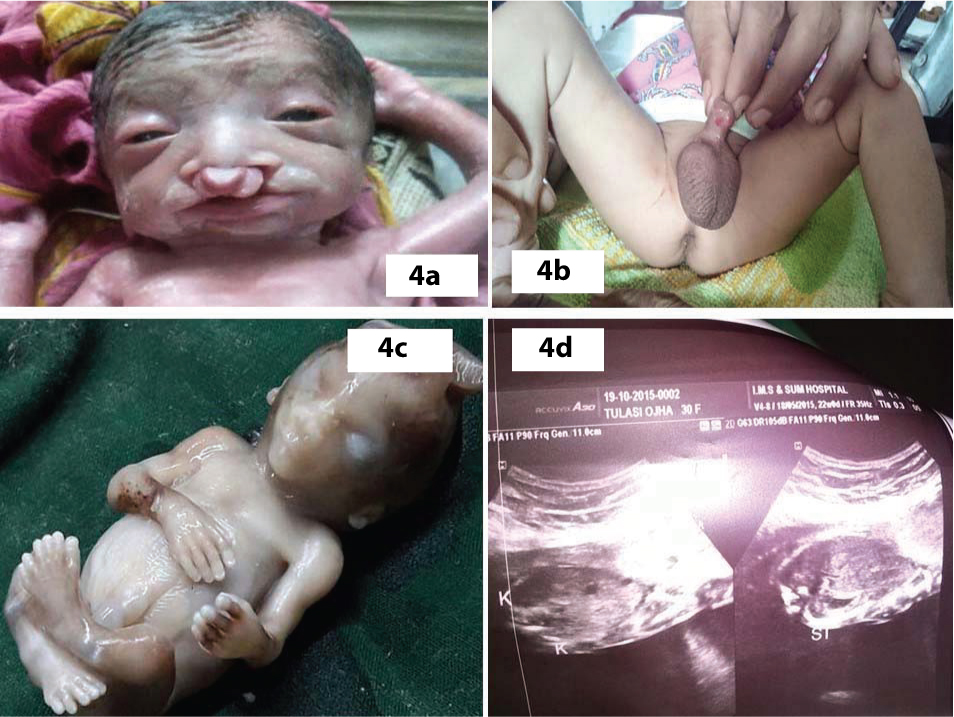
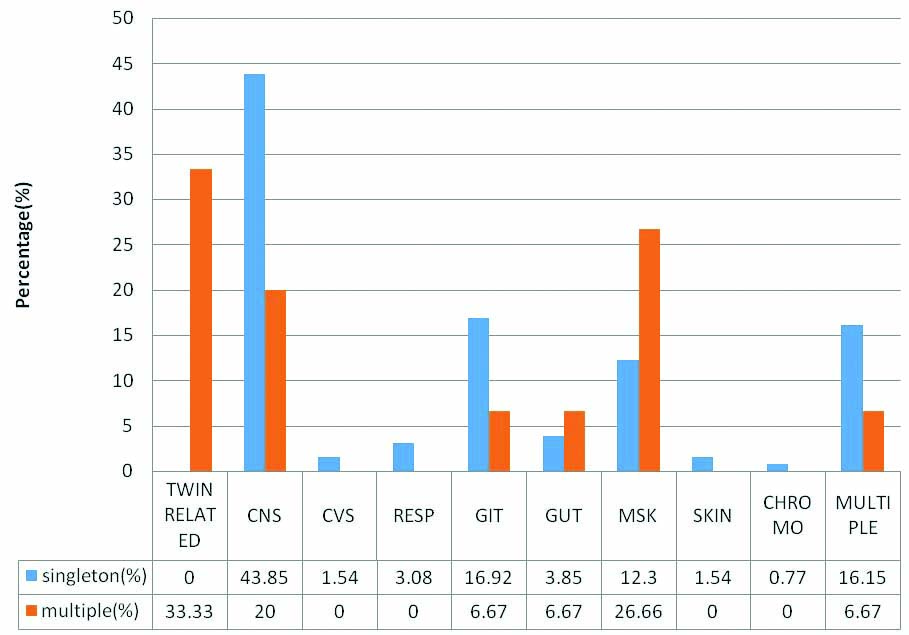
The details of anomalous babies in twin pregnancies and comparison of their incidence with that of singleton births are depicted in [Table/Fig-2]. There were five pairs of twin associated anomalies in which both babies were affected, one pair each of acardiac twin with Twin Reversal Arterial Perfusion (TRAP) sequence (female pair=FP, [Table/Fig-3a]), thoraco-omphalopagus twins (male pair –MP, [Table/Fig-3b]), thoracopagus twins (FP, [Table/Fig-3c]), fetus papyraceous (FP, [Table/Fig-3d]), twin-twin transfusion (FP, [Table/Fig-3a]). Among the three pairs of CNS anomalous twins, only one baby of the twins was affected and the anomalies were one anencephaly (FP, [Table/Fig-3e]), one encephalocele (MP, [Table/Fig-3f]), one meningomyelocele (FP). Among the orofacial and GIT affected anomalies, both the twins of one female pair was affected; one baby had cleft lip with cleft palate [Table/Fig-4a] and other baby had only cleft lip. Among the genitourinary [Table/Fig-4b], musculoskeletal [Table/Fig-4c] and multiple system (hydrops) anomalies, only one baby of each pair was affected. Most common malformation among multiple births seen in our study was twin associated malformations (5 out of 15 twin births=33.33%) followed by musculoskeletal defects, (4 out of 15 twin births=26.66%), CNS anomalies (20%), GIT, genitourinary and multiple system (6.67%) anomalies. Comparison of occurrence of anomalies between singleton and multiple births are shown in [Table/Fig-5]. In singleton births most common malformation is CNS anomalies (57 out of 130=43.85%), followed by GIT anomalies (16.92%), multiple systems affected anomalies (16.15%), musculoskeletal anomalies (12.30%). Less affected systems in singletons were genitourinary anomalies, respiratory system, CVS, skin and chromosomal anomalies, respectively.
Congenital malformation in multiple births.
| Serial number | Congenital anomaly | Number of affected babies | Sex of the baby | Gestational Age |
|---|
| Associated with twinning | Acardia with TRAP | 2 | FP | 20 weeks 6 days |
| Conjoint twin | 4 | MP(thoraco-omphal-opagus)FP(thora-copagus) | 38 weeks 1 day28 weeks 4 days |
| Fetus papyraceous | 2 | FP | 37 weeks 2 days |
| Twin-twin transfusion | 2 | FP | 24 weeks 1 day |
| Central Nervous System | Anencephaly | 1 | FP | 31 weeks 6 days |
| Encephalocele | 1 | MP | 38 weeks 1 day |
| Meningomyelocele | 1 | FP | 35 weeks 5 days |
| Orofacial and Gastrointenstinal system | Cleft lip with cleft palate | 1 | FP | 36 weeks |
| Cleft lip | 1 | |
| Genitourinary system | Hypospadiasis | 1 | MP | 38 weeks 3 days |
| Muscul-oskeletal system | Polydactyly | 1 | FP | 33 weeks 3 days |
| Syndactyly | 1 | M/1F | 32 weeks 3 days |
| Unilateral Congenital Talipes Equinovarus (CTEV) | 1 | FP | 34 weeks |
| Pectus excavatum | 1 | FP | 39 weeks |
| Multiple systems affected | Hydrops | 1 | FP | 33 weeks 3 days |
Photographs of anomalous twins: a) Acardia with TRAP sequence; b) Thoraco-omphalopagus; c) Thoracopagus; d) Fetus papiraceous; e) Anencephaly; f) Encephalocele.
a) Cleft lip and cleft palate; b) Hypospadia; c) Polydactyly; d) Ultrasonography of twin –twin transfusion.
Comparison of anomalies between singletons and multiple births.
[Table/Fig-6] depicts comparison of risk and demographic factors between cases and controls. Mean maternal age was 30.80 years in cases and 26.73 years in controls. But mean paternal age was 34 years and 28.07 years in cases and controls respectively which was statistically significant. In this study, majority of the cases were from a lower socioeconomic status (14 out of 15 cases, 93.33%) and rural habitat (13 of 15 cases, 86.66%). Female fetus was much more commonly affected (11 cases, 73.33% were FT pairs). Mean gestational age in cases was 33.47 weeks and in controls was 34.47 weeks. Four cases (46.67%) had history of fever in 1st trimester. Investigations in fever cases yielded neutrophilic leukocytosis and no other abnormalities. Six cases (40%) had addiction history (4 cases of passive smoking and 2 cases addicted to tobacco). There was significant p-value in lower socioeconomic status (p=0.018), rural habitat (p=0.004), female sex of affected fetuses (p=0.001), history of fever in 1st trimester (p=0.03), history of addiction (p<0.05). Fourteen babies out of 30 (15 twin births) were stillborn in cases but only 2 babies were stillborn in controls which was statistically significant (p<0.05). One case and four controls had folate deficiency, eight cases and seven controls were anaemic, one patient from case and three patients from control group had hyperlipidemia and only one case from control group had diabetes. So, it didn’t reveal any positive association with folate deficiency, anaemia, hyperlipidemia or diabetes mellitus. There was no significant difference in consanguinity, infertility treatment, previous history of birth defect, abortion, drug intake in 1st trimester. One mother from case group and seven from control group had hypertension and this revealed that in present study there was no positive association between birth defects and history of hypertension. Out of 15 cases of multiple births, in 3 cases the mothers had taken ovulation induction drugs and 1 case had taken ayurvedic medicines for infertilty. No one had used assisted reproduction techniques like In-Vitro Fertiliation (IVF) or Intra-Cytoplasmic Sperm Injection (ICSI).
Discussion
In the above study, total number of multiple births were less when compared to singleton births, but the prevalence of CAs in multiple births were more as compared to singleton births. The present study includes abortions more than 20 weeks, live births and still births. The probable reason could be many pregnancies with birth defects end up in early abortions (<16 weeks). This hospital is serving most of the rural pregnant women. The decrease in incidence of multiple births may be attributed to less use of ovulation inducing drugs. Most of the pregnant women were admitted as an emergency and may not have done ultrasonography.
The present data regarding the number of singleton and multiple births is smaller when compared with other studies but the present study shows higher occurrence of CAs compared with other studies, probably due to accuracy of diagnosis and proper documentation of the statistics and also the study being conducted in the referral care centre related to CAs. The prevalence of anomalies in multiple births, in the present study is 539.57/10,000 births which is higher than the incidence reported by Menasinkai SB et al., Zhang XH et al., Taksande A et al., [4,6,7]. But Sipek A et al., reported 598.38/10,000 births which is higher than reported in our study [8]. In a recent study done by Pattanaik T et al., in 2016 the prevalence of congenital malformation was 12.5% among singletons in a hospital based study which is higher than our finding [9]. Comparison of data among different authors is depicted in [Table/Fig-7].
Demographic and risk factors.
| Variables | Cases (n=15) | Control (n=15) | p-value (<0.05) |
|---|
| Maternal age (mean age in years) | 30.80 | 26.73 | NS |
| Paternal age (mean age in years) | 34 | 28.07 | 0.030 |
| Socioeconomic status | Low=14Middle=1High=0 | Low=8Middle=7High=0 | 0.018 |
| Habitat | Rural=13Urban=2 | Rural=5Urban=10 | 0.004 |
| Consanguinity | 0 | 1 | 0.5 |
| H/O birth defect | 2 | 0 | 0.2 |
| H/O still born | 3 | 0 | 0.1 |
| H/O spontaneous abortion | 3 | 1 | 0.299 |
| Gender | Male pair=3Female pair=111male1female=1 | Male pair=5Female pair=11male1female=9 | 0.001 |
| Gestational age (mean age in weeks) | 33.47 | 34.47 | NS |
| Anaemia | 8 | 7 | NS |
| Hyperlipedemia | 1 | 3 | NS |
| Fever in 1st trimester | 4 | 0 | <0.05 |
| Drug intake in 1st trimester | 2 | 0 | 0.143 |
| ↓ Serum folic acid | 1 | 4 | 0.142 |
| Diabetes mellitus | 0 | 1 | 0.309 |
| H/O hypertension | 1 | 7 | 0.013 |
| Addiction | 6 | 1 | <0.05 |
| Infertility treatment | 3 | 1 | 0.2 |
| Outcome | Live born=16Stillborn=14 | Live born=28Stillborn=2 | <0.05 |
NS=Not significant.
Comparison with other studies done in India.
| Authors | Singleton births | No of babies with CA | Incidence of CA/10,000 births | Multiple births | No of babies with CA | Incidence of CA/ 10,000 births |
|---|
| Taksande A [7] et al., | 9262 | 171 | 184.6 | 62 | 2 | 322.5 |
| Menasinkai SB [4] et al., | 48700 | 235 | 48.28 | 579 | 11 | 189.98 |
| Pattnaik T [9] et al., | 7973 | 100 | 1250 | x | x | x |
| Present Study | 10539 | 130 | 123.35 | 278 | 15 | 539.57 |
Despite the lack of zygosity determination some interesting assumptions could be made regarding fetal dysmorphology in SS and OS twins. Anomalies associated with twinning were substantially elevated in SS especially FP twins in our study, suggesting an increased risk limited to SS twins. In this study, 13 of 15 pairs are SS twins. Review of literature reveals that among 4490 twins, zygosity was not recorded and Weinberg formula applied, which showed that elevated risk appears limited to SS twins and is probably related to monozygosity. Considering twin associated anomalies, there are five cases in the present study. Menasinkai SB et al., reported four cases comprising of three acardiac twins and one thoracopagus among 11 CAs seen in 579 previous studies, reported four pairs of Siamese twins (MZ), one acardiac fetus with TRAP sequence, one fetus papyraceous, two hydroenencephaly [4]. Gupta P et al., reported one acardiac twin among nine CA seen in 133 twins [10].
In the present study, there were three cases among twin births with CNS anomalies, where as there are 57 cases among singleton births. Menasinkai SB et al., reported five cases of CNS anomalies {three Neural Tube Defect (NTD), two encephalocele} [4]. One CA with hydrolethalus syndrome (hydrocephalus, cleft lip and cleft palate) was reported by Gupta P et al., [10]. According to Glinianaia SV et al., incidence of CNS anomalies was 53.8/10000 in twin births; anencephaly and hydrocephalus were more than spina bifida in twin births [11]. In this study, there was no hydrocephalus.
In a large series, Hardin J et al., reported, that there were 4858255 singleton births with 14078 babies with CVS defects [12]. Among 54602 twins 628 babies had CVS defects. Increased prevalence was observed in twins compared to singletons in all 16 categories (ICD 10), According to Glinianaia SV et al., incidence of CVS Defects is 114.3/10,000 twin pregnancies as compared to 77.6/10,000 in singleton births [11]. CVS defects are not visible externally, so only those neonates who manifest symptoms early have been included and this might be the reason for under reporting of CVS defects.
Incidence of genitourinary anomalies in twins was 56.1/10,000 compared to 28.9/10,000 in singleton births as reported by Glinianaia SV et al., whereas in the present study there was only one case of hypospadiasis among multiple births and five cases among singleton pregnancies [11]. Review of literature has not definitely revealed a specific aetiology for a particular CA. Hence, it may be a random finding and may be geographic variation among study population. Gupta P et al., reported 4 club foot out of 9 babies with CA [10]. The incidence of Musculoskeletal (MSK) anomalies reported by Sipek A et al., is 90.93/10,000 [8]. Review of literature shows MSK was less common. It was 42.6/10,000 in twin pregnancies as compared to 19.8/10,000 in singleton births as observed by Glinianaia SV et al., [11]. In the present study, there were four out of 15 multiple births and 16 of 130 singleton births had MSK defects. There was an increased incidence of MSK anomalies among twin births. Frequency of chromosomal anomalies and skin disorders in multiple births are same or less than singleton births. Chromosomal analysis is difficult in these cases since most babies are stillborn and most parents in this study come from a lower socioeconomic status so either the parents can not afford or they do not want to spend for babies with such dismal prognosis.
The paternal age effect was first proposed implicitly by Weinberg in 1912 and explicitly by Penrose in 1955. In the current study, association between higher paternal age and CA was noted. Similar findings were noted in a study by Ghosh S et al., [13]. Mothers from a lesser deprived socioeconomic status and urban habitat must have gone for termination of pregnancy at GA (Gestational Age) <20 weeks, after antenatal detection (sonographically) and abortion <20 weeks were not included in the study so most of the cases were from a rural background and from a lower socioeconomic status. Similar findings have been reported by Smith LK et al., in their study [14].
Present study doesn’t reveal dominant role of ovulation inducing drugs or assisted reproduction techniques like IVF or ICSI in causation of CA. Only three out of 15 cases of multiple births had taken ovulation induction drugs and one case had taken ayurvedic medicines for infertilty. But no one had used assisted reproduction techniques. Literature reveals conflicting data regarding this. Minasinkai SB et al., had described that there is increase in twinning rate due to advanced maternal age, hereditary factors and use of ovulation inducing drugs and this results in premature and low birth weight babies associated with poor lung maturity [4]. However, other authors have opined contrary to this and have revealed that these drugs don’t have any relationship with incidence of birth defects. But a recent retrospective study conducted over a period of 9-years (2001-2011) in France, concluded that IVF pregnancies have a higher prevalence of major congenital malformations, with Adjusted Odds Ratio (AOR) of 2.0 {95% (CI) 1.0-3.8} and 2.0 (CI 1.3-3.1); 3.6 and 4.2% of infants born, respectively [15].
Female fetuses were predominantly affected in the present study. 73.33% were FPs which goes with the clinical findings of Kar A et al., [16]. However does not tally with the findings of the study done by Taksande A et al., which states that, male fetuses were more commonly affected [7]. In present study, history of fever in 1st trimester is statistically significant. Similar findings were reported by Ghosh S et al., [13]. In this study, there was no positive association with diabetes; similar findings were reported by Kar A et al., [16]. In this study, six out of 15 cases were exposed to some form of addiction, which was statistically significant. Similar findings have been reported by other authors also in last few decades. Here, out of 15 multiple births, 46.66% were stillborns. In the study of Shamnas M et al., congenital malformation contributes to 19.5% of perinatal mortality and 9.9% of stillbirths [17]; whereas Ghosh S et al., reported 37.6% stillborn among 130 malformed fetuses [13].
Present study reveals that none of the cases had history of CA in previous pregnancies or recurrence in subsequent pregnancies wherever they could be followed. In their study, Glinianaia SV et al., also observed higher recurrence risks for similar CA in isolated (20 folds) and syndromic (34 folds) groups where as it was low for dissimilar anomalies [18].
Conclusion
This study was conducted with an objective to know the demographic factors of birth defects in multiple births. Higher incidence of malformations has been detected among SS twins, especially FPs compared to OS and singleton births. Twin associated birth defects such as, acardiac twin with TRAP sequence and conjoint twins are common with MTs and at a higher risk of mortality and morbidity. Higher paternal age, lower socioeconomic status, rural habitat, fever in 1st trimester, addiction history such as intake of tobacco, passive smoking might be contributing factors. In the present scenario of small family norms and population control, greater emphasis should be given on early screening of birth defects by biochemical and sonographic tests in the first trimester because the co-occurrence of multiple birth and congenital anomaly among live born infants places a huge burden on parents and health care sectors.
NS=Not significant.