A Case Report of Rituximab Induced Angioedema
Dhaivat Shukla1, Karan Shah2, Sapan Pandya3, Supriya Malhotra4, Pankaj Patel5
1 Fellow, Department of Rheumatology, V.S General Hospital, Ahmedabad, Gujarat, India.
2 2nd Year Resident, Department of Pharmacology, Smt. NHL Municipal Medical College, Ellisbridge, Ahmedabad, Gujarat, India.
3 Professor and Head, Department of Rheumatology, V.S General Hospital, Ahmedabad, Gujarat, India.
4 Professor and Head, Department of Pharmacology, Smt. NHL Municipal Medical College, Ellisbridge, Ahmedabad, Gujarat, India.
5 Dean, Smt. NHL Municipal Medical College, Ellisbridge, Ahmedabad, Gujarat, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Karan Shah, 2nd Year Resident, Department of Pharmacology, Smt. NHL Municipal Medical College, Ellisbridge, Ahmedabad-380006, Gujarat, India.
E-mail: karannhl@hotmail.com
Rituximab is a genetically engineered chimeric murine/human monoclonal IgG1 kappa antibody directed against the CD20 antigen. It has been approved for rheumatoid arthritis and is a promising new agent in the treatment of several autoimmune neurological disorders and also Sjögren’s syndrome. Common adverse reactions to treatment with Rituximab include infusion reactions including angioedema and hypersensitivity reactions. In the present case, we observed severe angioedema after low dose of Rituximab therapy. Clinicians should be vigilant when using Rituximab for such hypersensitivity phenomenon occurring despite pre-medicating with glucocorticoids.
Hypersensitivity reactions, Rheumatology, Sjögren’s syndrome
Case Report
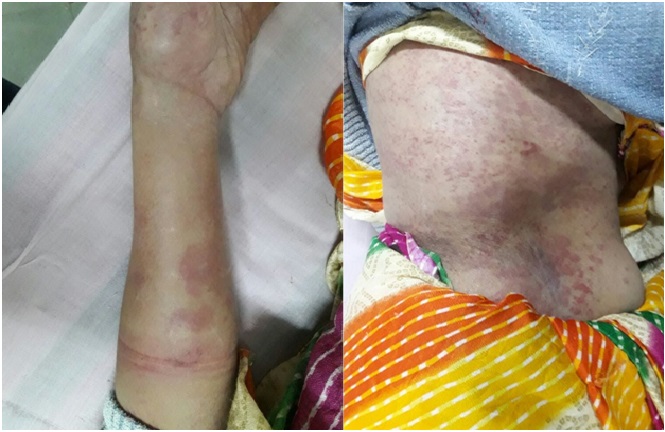
A 60-year-old female presented to the Department of Rheumatology, Sheth V.S. General Hospital, Ahmedabad, India, with the chief complaints of dry mouth, fatigue and pain in joints for a week. She was a known case Sjögren’s syndrome for seven years. She was taking Tab. Hydroxychloroquine 200 mg once daily, Tab. Prednisone 5 mg once daily and Tab. Folic Acid 5 mg once daily for the same. Despite being on this treatment, her ESSDAI (EULAR Sjögren’s syndrome disease activity index) score was 13 and she was not relieved of her symptoms [1]. She was admitted and infused with Rituximab 100 mg slowly intravenously over three hours. Premedication in the form of Methylprednisolone was administered before starting Rituximab infusion. She was discharged the same day. Patient returned the next day with the chief complaints of severe itchy rash all over the body. There were multiple, small, well defined, annular, non-tender, non-scaly, non-blanchable erythematous plaques with few superficial erosions over chest, abdomen and left forearm [Table/Fig-1]. This was thought to be angioedema mostly due to Rituximab. It was managed with Hydrocortisone 100 mg and Pheniramine maleate 22.75 mg/2 mL stat. Patient recovered after the same. This Adverse Drug Reaction (ADR) was reported to nearest PvPI (The Pharmacovigilance Program of India)-ADR Monitoring Centre with Report id 2017-64381. After recovery, the patient was started on Tab. Hydroxychloroquine 300 mg once daily and Tab. Prednisolone 20 mg once daily. Patient is symptomatically better since then.
Clinical picture of the patient.
Discussion
Rituximab is a genetically engineered chimeric murine/human monoclonal IgG1 kappa antibody directed against CD20 antigen [2]. Complement-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity are direct effects and structural changes, apoptosis, and sensitisation of cancer cells to chemotherapy are indirect effects of Rituximab [2]. Rituximab appears to be effective in several autoimmune disorders by causing B-cell depletion [3]. Latest treatment guidelines for Sjögren’s syndrome are suggestive of label use of Rituximab as a therapeutic option in patients with primary Sjögren’s syndrome for whom conventional therapies have proven insufficient [4]. This is based on category B level of evidence and class IIb strength of recommendation. Significant number of Rituximab-associated serious adverse events affecting various systems has been reported [5]. The incidence of angioedema caused by intravenous immunoglobulin therapy is 11% [6]. Serious and sometimes fatal complications of Rituximab including grade 3 and 4 adverse reactions have also been reported in 1% of patients [7]. Angioedema is a potentially serious side effect of Rituximab therapy. The aetiology of angioedema caused by Rituximab is explained by ‘cytokine release syndrome’ that has been described after Rituximab infusions [8]. ‘Cytokine release syndrome’ is a symptom complex associated with the use of many monoclonal antibodies. It results from the release of cytokines from cells targeted by the antibody as well as immune effector cells recruited to the area. When cytokines are released into the circulation, systemic symptoms such as fever, nausea, chills, hypotension, angioedema, tachycardia, asthenia, headache, rash, scratchy throat, and dyspnea can result [9]. Previous studies suggest that the frequency of infusion reactions, which form the bulk of major ADRs due to Rituximab administration, decreased in patients who were premedicated with intravenous glucocorticoids. In contrast with the same, our patient had the ADR despite being pre medicated with glucocorticoid [10]. Usually infusion reactions occur within 24 hours of the infusion and are dose dependent [11]. In this case it happened after seven hours of infusion but the dose of Rituximab was much lesser than recommended (100 mg instead of 500 mg). In one similar incidence, a patient developed serum sickness after the first administration of Rituximab and severe angioedema after the second dose [12].
Conclusion
Rituximab can cause angioedema despite premedication with glucocorticoids. So, clinicians should be vigilant towards such hypersensitivity phenomenon while using Rituximab.
[1]. Seror R, Bowman S, Brito-Zeron P, Theander E, Bootsma H, Tzioufas A, EULAR Sjogren’s syndrome disease activity index (ESSDAI): a user guideRMD Open 2015 1(1):00002210.1136/rmdopen-2014-00002226509054 [Google Scholar] [CrossRef] [PubMed]
[2]. Rituxan (Rituximab): Side Effects, Interactions, Warning, Dosage & Uses. Retrieved from RxList [Google Scholar]
[3]. Kosmidis M, Dalakas M, Practical considerations on the use of rituximab in autoimmune neurological disordersTher Adv Neurol Disord 2010 3(2):93-105.10.1177/175628560935613521179602 [Google Scholar] [CrossRef] [PubMed]
[4]. Carsons S, Vivino F, Parke A, Carteron N, Sankar V, Brasington R, Treatment guidelines for rheumatologic manifestations of sjögren’s syndrome: use of biologic agents, management of fatigue, and inflammatory musculoskeletal painArthritis Care Res 2017 69(4):517-27.10.1002/acr.2296827390247 [Google Scholar] [CrossRef] [PubMed]
[5]. Retrieved from VigiAccessTM Database [Google Scholar]
[6]. Retrieved from Drugs@FDA, US Food and Drug Administration [Google Scholar]
[7]. Schwartzberg L, Stepanski E, Walker M, Mathias S, Houts A, Fortner B, Implications of IV monoclonal antibody infusion reaction for the patient, caregiver, and practice: results of a multicenter studySupport Care Cancer 2008 17(1):91-98.10.1007/s00520-008-0474-518592276 [Google Scholar] [CrossRef] [PubMed]
[8]. Jensen M, Winkler U, Manzke O, Diehl V, Engert A, Rapid tumor lysis in a patient with B-cell chronic lymphocytic leukemia and lymphocytosis treated with an anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab)Ann Hematol 1998 77(1-2):89-91.10.1007/s0027700504199760161 [Google Scholar] [CrossRef] [PubMed]
[9]. Breslin S, Cytokine-release syndrome: overview and nursing implicationsClin J Oncol Nurs 2007 11(0):37-41.10.1188/07.CJON.S1.37-4217471824 [Google Scholar] [CrossRef] [PubMed]
[10]. Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: Results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trialArthritis Rheum 2006 54(5):1390-400.10.1002/art.2177816649186 [Google Scholar] [CrossRef] [PubMed]
[11]. Ghielmini M, Rufibach K, Salles G, Leoncini-Franscini L, Léger-Falandry C, Cogliatti S, Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK)Ann Onco 2005 16(10):1675-82.10.1093/annonc/mdi32016030029 [Google Scholar] [CrossRef] [PubMed]
[12]. Kumar A, Khamkar K, Gopal H, Serum sickness and severe angioedema following rituximab therapy in RAInt J Rheum Dis 2011 15(1):6-7.10.1111/j.1756-185X.2011.01645.x22324965 [Google Scholar] [CrossRef] [PubMed]