Carcinosarcomas of the uterus are rare, highly aggressive forms of biphasic tumours. These are monoclonal in origin with disproportionate percentage of mortality. This entity comprises of admixture of carcinomatous and sarcomatous elements. There are two histological subtypes based on the the sarcomatous components which can be either hetrerologous or homologous. In majority of uterine carcinosarcomas both the carcinomatous and sarcomatous elements are high grade. These can be associated with mesenchymal epithelial transition presenting as Microcystic Elongated and Fragmented Pattern (MELF) which is a rare and potential mimicker of vascular invasion. A, 62-year-old, female presented with complaints of vaginal bleeding and discharge for three months with a polypoidal mass in the cervix. Histopathology revealed a biphasic tumour consisting of epithelial and mesenchymal elements. Immunohistochemistry for PAN-CK, CK 19, p63, CEA, EMA, vimentin, ER, PR, Desmin, S100, SMA, CD 34 and CD10 along with HPV DNA on cervical swab was performed. Final diagnosis of carcinosarcoma with MELF was conferred. The case was highlighted due to its rarity and unique histological pattern.
Case Report
A, 62-year-old obese female presented with chief complaints of post menopausal bleeding with discharge for three months with a polypoidal mass in the cervix on per speculum examination. The patient was a known case of diabetes for last 10 years and was on oral hypoglycaemics.
Haematological profile was within normal limits. Fasting blood glucose and glycosylated haemoglobin were normal. A provisional diagnosis of cervical fibroid was made clinically.
Positron Emission Tomography-Computed Tomography (PET-CT) revealed a large, lobulated heterogeneously enhancing, hyper metabolic mass lesion involving cervix and enchancing Fludeoxyglucose (FDG) avid thickening of the endometrium. Multiple enlarged hypermetabolic precaval, right common iliac, and bilateral pelvic lymph nodes with multiple avid nodules in both lungs were noted. Also, seen FDG avid consolidation in paramediastinal upper lobe of left lung along with diffuse thickening of the omentum, these were likely to be metastatic.
Biopsy was performed. Gross specimen examination revealed grey white soft tissue bits; largest measuring 4x3x3 cm and smallest measuring 0.5x0.5 cm. Cut section is homogenous grey white with few haemorrhagic areas.
Microscopy showed tumour cells arranged in glandular pattern, sheets, and clusters and singly dispersed having marked degree of pleomorphism. The cells had high nuclear cytoplasmic ratio with prominent nucleoli. Also seen were bizarre cells which were binucleate and multinucleate in the stroma against a myxoid background [Table/Fig-1a]. Foci of fragmented glands were also noted [Table/Fig-1b]. Large areas of coagulative necrosis with extensive vascular proliferation were seen. Mitotic figures were 5 to 10 per High Power Field (HPF) [Table/Fig-2]. Diagnosis of high grade malignant lesion possibly carcinosarcoma of the uterus or cervix was proposed on histopathology.
a) Showing atypical cells having high nuclear cytoplasmic ratio, hyperchromasia forming gland like structures with bizzarecells in the stroma against a myxoid background (H&E, Magnification 20X); b) Showing fragmented glands of MELF pattern (H&E, Magnification 20X).
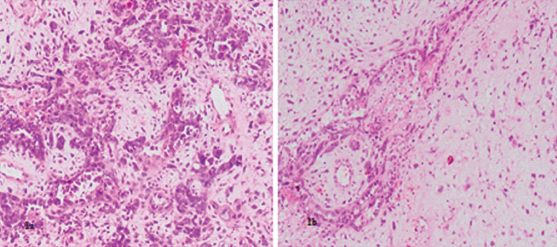
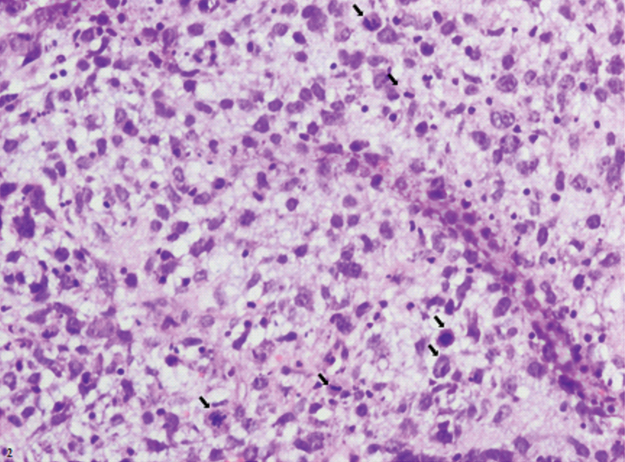
Figure showing areas of coagulative necrosis with extensive vascular proliferation. Arrows showing mitotic figures were 5 to 10 per high power field (H&E, Magnification 40X).
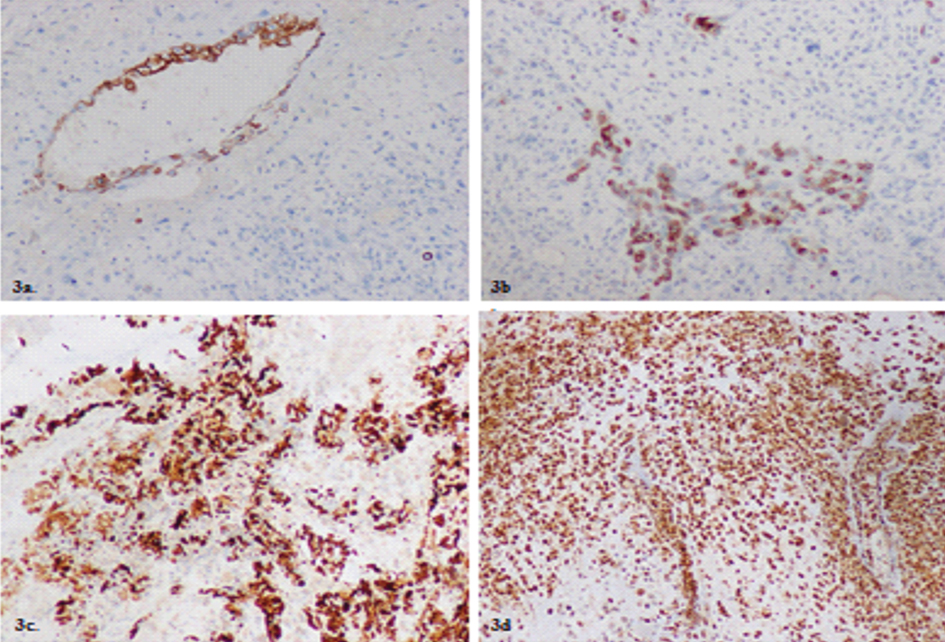
Markers advised were Pan CK, CK 19, p63, CEA, EMA vimentin, ER, PR, Desmin, S100, SMA, CD 34 and CD10 along with Human papilloma virus (HPV) DNA on cervical swab to confirm origin of the tumour. IHC revealed CK–19 [Table/Fig-3a], EMA [Table/Fig-3b] and PanCK [Table/Fig-3c] positivity revealing the epithelial component of the tumour. CEA showed occasional cytoplasmic positivity [Table/Fig-3d].
a) Showing CK 19 positivity on IHC in the glandular components of the tumour (Magnification 20X); b) Showing positivity on IHC for EMA for glandular component (Magnification 20X); c) Showing PAN-CK positivity in glandular component (Magnification 40X); d) Showing occasional cytoplasmic CEA positivity in tumour cells (Magnification 20X).
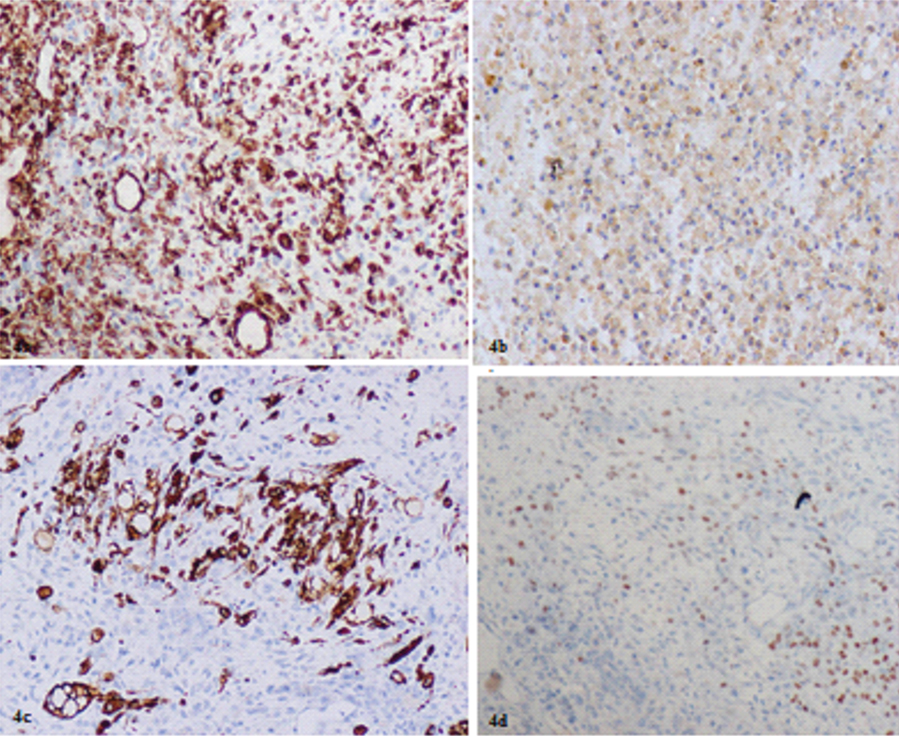
Vimentin was positive in stromal cells and few epithelial cells [Table/Fig-4a]. Occasional stromal cells showed CD 10 positivity [Table/Fig-4b] along with Desmin [Table/Fig-4c] featuring the sarcomatous component of the tumour. p63 [Table/Fig-4d] showed focal nuclear positivity in poorly differentiated tumour cells. Rest of markers and HPV cervical swab was negative.
a) showing both the stromal cells and few epithelial cells positive for Vimentin (Magnification 20X); b) showing occasional CD 10 positivity in tumour cells (Magnification 40X); c) showing occasional stromal cells positive for Desmin (Magnification 40X); d) showing focal nuclear positivity for p63 on IHC in poorly differentiated tumour areas (Magnification 20X).
The patient was referred based on the histopathology PET scan report in a oncology centre and advised for laparotomy followed by total abdominal hysterectomy with bilateral salpingoophorectomy. However, the case was lost to follow up.
Discussion
Uterine Carcinosarcomas (CS) also known as Malignant Mixed Mullerian Tumours (MMMT) are highly aggressive, rare, biphasic tumours first described by Ferreria in 1951 [1]. It accounts for less than 4% of all uterine cancers [2]. The CS of uterus with MELF pattern is rare with high propensity for lymph node invasion [3]. In CS, carcinomatous component is most commonly serous, clear or high-grade endometrioid, while the sarcomatous component may display a wide range of morphology, such as leiomyosarcoma and rhabdomyosarcoma, but is generally poorly differentiated [4].
Risk factors for CS are advanced age, nulliparity, obesity, exogenous estrogens and long term use of tamoxifen [5]. It typically presents with pyometra, vaginal bleeding, bloody or watery discharge, abdominal pain, or as a polypoid mass in a postmenopausal woman [6].
The present case presented with postmenopausal bleeding, discharge, pain with a fleshy polypoidal mass protruding from internal OS since three months in a 62-year-old obese female. On physical examination, 50%–95% of patients have enlargement of the uterus with 50% of patients having protrusion of a polypoid lesion through the endocervical canal [7]. The “symptom triad” indicative of carcinosarcoma includes pain, severe vaginal bleeding, and the passage of necrotic tissue per vaginum [8].
The current hypothesis for origin of CS is monoclonal origin where in a single clone of cells differentiates into epithelial (carcinomatous) and the stromal (sarcomatous) components [9]. Uterine carcino-sarcomas are mixed tumours with both components being malignant [10]. Higher incidence is found in blacks compared to white woman [11].
Recent studies indicates that the histological features of the stromal component including grade, mitotic index, and the presence or absence and types of heterologous elements bears no relation with the metastases or overall prognosis. In contrast, epithelial elements comprising of high grade carcinomas, serous or clear cell components are associated with a higher frequency of metastases, deep myometrial or cervical involvement and lymphatic or vascular space invasion. All these parameters are indicative of an aggressive behaviour [12]. When the uterine carcinosarcoma metastatise outside the uterus it is the epithelial component which is likely to be present in the implant [4].
This case report highlights primarily on the pattern of endometrial carcinoma which has areas of MELF, which is a recent concept of epithelial mesenchymal transition. MELF is frequently accompanied by a fibromyxoid or inflammatory stroma [13]. MELF invasion appears to be restricted to the low grade myoinvasive carcinomas of endometriod type and is more common in tumours exhibiting focus of mucinous differentiation. This suggests MELF represents a specific tumour stromal interaction rather than a degenerative process [14].
CK 19 and Vimentin both are positive in the present case indicating the tumour is biphasic in origin. IHC of our present case showed CK 19 with Vimentin positivity indicating the tumour is carcinosarcoma. CK 19 positivity in those areas of the tumours which on histopathology appeared like vascular invasion proved that these are of epithelial origin and not vascular.
These actually represented the fragmented glands of microcystic elongated fragmented pattern seen in endometriod carcinoma of the uterus. EMA, CK 19 and Vimentin positivity and ER and PR negativity along with negative HPV DNA in Cervical swab showed the carcinosarcoma is arising from uterus and is not of cervical in origin [15].
Sarcomatous component of the tumour was immunoreactive for CD10 and desmin. It supported that the component is possibly of myoid origin native to the uterus and hence the stromal component is homologous. Typically the carcinosarcomas are tumours composed of high-grade sarcomatous and carcinomatous elements [11].
Although this tumour met the criteria for CS, the histologic findings were very unusual with MELF pattern as these are unusual features in the epithelial component that is endometrioid, in this case of CS. The association of MELF with low grade endometriod carcinoma along with homologous stromal component makes this carcinosarcoma low grade and therefore a rare presentation. MELF invasion has been associated with nonvaginal recurrences and Lymph Node (LN) metastases [3]. The patient already had distant metastasis at the time of presentation as reported in PET scan.
CS is a Type II epithelial endometrial malignancy with high rates of p53 mutations and presents at an advanced stage and has poor prognosis [10]. Hysterectomy with bilateral salpingoophorectomy remains the main stay of treatment [2]. However, the recurrences can be present in 50 percent cases following surgery [6].
Conclusion
The epithelial and sarcomatous components are both usually high grade in CS. However, the presence of MELF pattern in carcinosarcoma, as in this case classifies it as Type 1 endometrial carcinoma. The sarcomatous component in present case is also homologous. The present case was a rare finding as carcinosarcomas are usually type 2 endometrial carcinomas. The presence of the MELF pattern caries a good prognosis.
Possibility of carcinosarcoma should always be considered by the clinician and surgeon in case of vaginal bleeding and cervical mass in postmenopausal women for appropriate surgical management on confirmation by histopathology and IHC.
[1]. Ferriera HP, A case of mixed mesodermal tumour of the uterine cervixJ obstet Gynaecol Br Emp 1951 58:446-48.10.1111/j.1471-0528.1951.tb04022.x14861690 [Google Scholar] [CrossRef] [PubMed]
[2]. Arend R, Doneza JA, Wright JD, Uterine carcinosarcomaCurr Opin Oncol 2011 23(5):531-36.10.1097/CCO.0b013e328349a45b21743326 [Google Scholar] [CrossRef] [PubMed]
[3]. Joehlin-Price AS, McHugh KE, Stephens JA, Li Z, Backes FJ, Cohn DE, The Microcystic, Elongated, and Fragmented (MELF) pattern of invasion: a single institution report of 464 consecutive FIGO grade 1 endometrial endometrioid adenocarcinomasAm J SurgPathol 2017 41(1):49-55.10.1097/PAS.000000000000075427740968 [Google Scholar] [CrossRef] [PubMed]
[4]. de Jong RA, Nijman HW, Wijbrandi TF, Reyners AK, Boezen HM, Hollema H, Molecular markers and clinical behavior of uterine carcinosarcomas: focus on the epithelialtumour componentMod Pathol 2011 24(10):1368-79.10.1038/modpathol.2011.8821572397 [Google Scholar] [CrossRef] [PubMed]
[5]. Ma CJ, Yang SF, Huang CC, Chai CY, Cheng KI, Tsai EM, Malignant mixed mülleriantumour of primary mesenteric origin associated with a synchronous ovarian cancer: Case report and literature reviewEur J Gynaecol Oncol 2008 29:289-93. [Google Scholar]
[6]. El-Nashar SA, Mariani A, Uterine CarcinosarcomaClinical Obstetrics and Gynecology 2011 54(2):292-304.10.1097/GRF.0b013e31821ac63521508698 [Google Scholar] [CrossRef] [PubMed]
[7]. Kuyumcuoğlu U, Kale A, Homologous type of malignant mixed mullerian tumour of the uterus presenting as a cervical massJournal of the Chinese Medical Association 2009 72:533-35.10.1016/S1726-4901(09)70423-X [Google Scholar] [CrossRef]
[8]. Doss LL, Llorens AS, Henriquez EM, Carcinosarcoma of the uterus: a 40 year experience from the state of MissouriGynecologic Oncology 1984 18(1):43-53.10.1016/0090-8258(84)90005-2 [Google Scholar] [CrossRef]
[9]. Clearman T, Cimic A, Ellenson LH, Gupta D, Clinically aggressive “low-grade” uterine carcinosarcoma: A case reportGynecol Oncol Rep 2015 14:09-11.10.1016/j.gore.2015.08.00226793763 [Google Scholar] [CrossRef] [PubMed]
[10]. Adachi Y, Nonogaki H, Minamiguchi S, Li M, Ikehara S, Carcinosarcoma of the uterus: A case reportMol Clin Oncol 2016 4(4):571-73.10.3892/mco.2016.74427073665 [Google Scholar] [CrossRef] [PubMed]
[11]. Brooks SE, Zhan M, Cote T, Baquet CR, Surveillance, epidemiology and end results analysis of 2677 cases of uterine sarcoma; 1989-1999Gynecol Oncol 2004 93:204-08.10.1016/j.ygyno.2003.12.02915047237 [Google Scholar] [CrossRef] [PubMed]
[12]. McCluggage WG, Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomasJ Clin Pathol 2002 55(5):321-25.10.1136/jcp.55.5.32111986333 [Google Scholar] [CrossRef] [PubMed]
[13]. Zaino RJ, Unusual patterns of endometrial carcinoma including MELF and its relation to epithelial mesenchymal transitionInternational Journal of Gynecological Pathology 2014 33(4):357-64.10.1097/PGP.000000000000013724901395 [Google Scholar] [CrossRef] [PubMed]
[14]. Stewart CJ, Brennan BA, Leung YC, Little L, MELF pattern invasion in endometriod carcinoma: association with low grade, myoinvasive endometiod tumours, focal mucinous differentiation and vascular invasionPathology 2009 41(5):454-59.10.1080/0031302090304113519900084 [Google Scholar] [CrossRef] [PubMed]
[15]. Stewart CJ, Crook ML, Lacey J, Louwen K, Cytokeratin 19 expression in normal endometrium and in low-grade endometrioid adenocarcinoma of the endometriumInt J Gynecol Pathol 2011 30(5):484-91.10.1097/PGP.0b013e318215894421804393 [Google Scholar] [CrossRef] [CrossRef] [PubMed]