The incidence of fungal infections has markedly increased in recent years particularly due to greater use of immunosuppressive drugs; prolonged use of broad-spectrum antibiotics; widespread use of indwelling catheters; and the Acquired Immunodeficiency Syndrome (AIDS). Fungal infections have emerged as a major cause of death among cancer and transplant recipients [1]. In addition, immunocompromised patients often experience more frequent and severe fungal infections. Most fungi that are pathogenic for humans are saprophytes in nature; they cause infection when airborne spores reach the lung or paranasal sinus or when hyphae or spores are accidentally inoculated into the skin or cornea. Most fungi infect hosts preferentially by one route and only infrequently by another routes. Candida albicans, a normal commensal in the mouth and intestine, reaches deeper tissues only when mucosal or cutaneous barriers are breached by disease, surgery, trauma or catheterization [2]. Some fungi, such as Aspergillus are said to be opportunists in that they usually infect hosts with compromised immunity [3]. Skin and soft tissue infections are the common sites of infection in immunocompromised hosts and usually pose a major diagnostic challenge. It is important to differentiate between bacterial and fungal infection in such cases because the successful management of such cases depend upon early recognition, timely surgical debridement or drainage and appropriate antifungal therapy. So, keeping all these factors in mind, the aim of the present study is: 1) to study the type of fungal isolates from pus and soft tissue specimens; 2) to clinically correlate these fungal isolates; 3) to study antifungal susceptibility profile of various Candida species.
Materials and Methods
The study was conducted at Indraprastha Apollo Hospitals, New Delhi, India. A retrospective analysis of the fungal isolates from pus/tissue specimens was done over a time period of one year i.e., September 2013 to August 2014. All the clinical data of the patient was collected from Medical Record department of the hospital with respect to demographic details, immune status, underlying co-morbid conditions, any surgical procedure, treatment and follow-up. All the pus/tissue specimens received in the Department of Microbiology for fungal culture were processed as per standard protocol. The tissue specimens comprised of any type of skin and subcutaneous tissue, nasal polyps/paranasal sinus tissue, other soft tissue specimens from visceral organs like Pancreas. The details of the type of specimen and underlying condition have been described in [Table/Fig-1]. Direct microscopic examination (KOH mount) of the specimens was done and the fungal culture was put on two types of Sabouraud’s Dextrose Agar (SDA) slants (SDA with and without Gentamycin- Chloramphenicol- cycloheximide) in duplicate and incubation of both types of SDA slants was done at 25°C and 37°C. The two types of SDA were prepared in house from dehydrated culture powder (HiMedia, Mumbai, India). Gentamycin (HiMedia, Mumbai, India), chloramphenicol (HiMedia, Mumbai, India) and cycloheximide (Sigma Aldrich, US) were added in SDA in concentration of 5 μg/mL, 16 μg/mL and 0.5 μg/mL respectively to inhibit the growth of bacteria and environmental moulds. The significance of the fungal growth was determined by clinical history and correlation with direct microscopic examination. Only that growth was considered to be significant in which direct microscopic examination showed presence of fungal elements (hyphae/yeast) along with clinical correlation. The fungal growth was identified by macroscopic and microscopic examination using Lactophenol Cotton Blue Mount (LPCB) examination. The yeast identification was done by automated methods using Vitek-2 compact (software version 7.01, Biomerieux, France) or MALDI-TOF Vitek-MS (software version V2, Biomerieux, France) and correlated with conventional methods like germ tube test, Chrome agar (Hi Media, India) and cornmeal agar. For Germ tube test, yeast growth was inoculated in 0.5 ml of pooled human serum followed by incubation at 37oC for 2- 3 hours followed by microscopic examination of wet mount at 40X objective. The yeast growth was deeply streaked in three parallel lines 1.5 cm long and about 1 cm apart on Corn meal Tween agar culture plate and a sterile cover slip was placed on it. The incubation was done at 25oC and was examined microscopically for chlamydospore formation and growth morphology after 48-72 hours along the edge of the coverslip in low and high power objectives. The mycelial fungi were identified by LPCB mount examination of the fungal growth on SDA slants and/or slide culture. The antifungal susceptibility testing of the yeast isolates were done by determining Minimum Inhibitory Concentration (MIC) for various drugs using Vitek-2 AST-YS06 cards (Biomerieux, France) and/or ATB FUNGUS 3 strips (Biomerieux, France) and/or E-test (Biomerieux, France) using RPMI agar. These methods of antifungal susceptibility testing had already been validated at our centre by comparing it with microbroth dilution technique. So, the interpretation of the results was done as per CLSI guidelines on broth dilution antifungal susceptibility testing of yeasts [4]. For Quality assurance of antifungal susceptibility testing methods, Candida glabrata ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality control strains. It was also tried to correlate the fungal culture growth with fungal KOH mount, and histopathological examination. A clinical correlation was also done with respect to age, gender, underlying disease, immune status, treatment and outcome.
Frequency of isolation of fungal isolates from pus/tissue specimens with respect to clinical details of the patients.
| Fungal isolates | Number (%) | Site of specimen | Underlying disease/Diagnosis | Treatment | Outcome |
|---|
| Candida albicans | 09 (24.3) | Abdominal pus | Post cholecystectomy-subdiaphragmatic collection | Ultrasound guided tube drainage of pus and Fluconazole | Discharged in stable condition |
| Wound discharge post Coronary Artery Bypass Grafting (CABG) | Coronary artery disease, Chronic Renal disease | Fluconazole | Cured |
| Ulcers on glans penis | Pyrexia of unknown origin with autoimmune disease | Voriconazole | Cured |
| Aspirate from retropharyngeal mass | Skull base osteomyelitis | Amphotericin B followed by Voriconazole | Discharged in stable condition |
| Oral ulcer | Dermatomyostis with facial cellulitis | Local application of clotrimazole | Recurrence |
| Abdominal wound | Ileal stricture and perforation peritonitis | Amphotericin B | Expired |
| Right lung empyema | Chronic renal disease, right lower lobe consolidation | Ultrasound guided pleural fluid drainage and Amphotericin B followed by Voriconazole | Cured |
| Pus from groin and buttocks | Bilateral lower limb cellulitis on antibiotics | Daily dressing and local application of clotrimazole | |
| Trophic ulcer right foot | Ulcer right foot, Coronary artery disease | Debridement | Discharged in stable condition |
| Candida glabrata | 08 (21.6) | Ulcerative blisters all over the body | Chronic renal disease on Haemodialysis | Diagnosed as Toxic Epidermal Necrolysis,Local application of Fluticasone and oral Voriconazole | Expired |
| Abdominal wound | Post operative case of bowel ischaemia with Faecal peritonitis and anastomotic leak | Anidulafungin | Discharged on request (Poor prognosis predicted) |
| Necrotic Pancreatic tissue | Traumatic Pancreatitis | Pancreatic necrosectomy with FJ | Discharged in stable condition |
| Abdominal wound discharge | Adenocarcinoma duodenum with whipple’s procedure done | Caspofungin | Cured |
| Intraabdominal abscess | Decompensated chronic liver disease | Drainage and Caspofungin | Expired |
| Tongue ulcer | Post chemotherapy mucositis post renal transplant, metastatic carcinoma | Local application of Clotrimazole | Ulcers subsided |
| Abdominal pus | Follow up case of carcinoma colon, Peripheral vascular disease | Drainage and Caspofungin | Discharged in stable condition |
| Abdominal wound discharge | Disseminated tubercular perforation peritonitis | Caspofungin | Discharged |
| Candida tropicalis | 03 (8.1) | Liver abscess | Gall stone disease with choledocholithiasis, hepatic abscess | Ultrasound guided drainage of hepatic abscess followed by Voriconazole, Common bile duct exploration | Cured |
| Burn wound swab | Burns with sepsis, acute kidney and lung injury | Daily dressing | Discharged in stable condition |
| Wound sinus on face | Road traffic accident with facial injury | Voriconazole | Discharged in stable condition |
| Candida haemulonii/auris | 03 (8.1) | Subglial granulation tissue | Post cranioplasty infected bone implant | Re-exploration of left fronto-parieto-temporo skin flap with removal of infected brain cement and granulation tissue | Wound healthy at the time of discharge |
| Wound bilateral cheek | Road traffic accident with head injury with polytrauma | Daily dressing | Cured |
| Slough on under surface of tibia and femur | Infected Total knee replacement (TKR) | Revision TKR Voriconazole/Amphotericin B | Recurrence till date with isolation of Candida haemulonii/auris |
| Candida pulcherimma | 01 (2.7) | Multiple ulcers over left leg | Peripheral vascular disease | Voriconazole | Improved |
| Dematiaceous fungi | 05 (13.5) | Left ankle infected cyst(Exophiala sp.) | Post Renal transplant | Excision of cyst followed by Itraconazole | Improved |
| Abscess right foot(Exophiala sp.) | Post Renal transplant | Incision and drainage followed by Ketoconazole | Improved |
| Left parieto-occipital abscess(Cladophialophora sp.) | Post Liver transplant | Craniotomy followed by Itraconazole | Improved and discharged in stable condition |
| Right ankle swelling(Exophiala sp.) | Renal transplant | Incision and drainage followed by Voriconazole | Improved |
| Intracranial abscess(Cladophialophora sp.) | Post renal transplant | Craniotomy and decompression followed by Itraconazole | Expired |
| Aspergillus spp. | 07 (18.9) | Nasal mucosa(Aspergillus flavus) | Rhinosinusitis | Functional endoscopy Sinus Surgery (FESS) | Improved |
| Wound pus on face(Aspergillus fumigatus) | Gunshot injury | Daily dressing and Voriconazole | Improved |
| Ethmoidal cell mucin (Aspergillus flavus) | Rhinosinusitis | FESS | Improved |
| Nasal polyp(Aspergillus flavus) | Nasal Polyposis | Polypectomy | Improved with no recurrence in one year follow up |
| Scrotal abscess sinus(Aspergillus fumigatus) | Scrotal abscess | Excision of Scrotal sinus | Improved |
| Nasal polyp(Aspergillus flavus) | Rhinosinusitis | Polypectomy | Improved |
| Tissue from maxillary sinus(Aspergillus flavus) | Rhinosinusitis | FESS followed by Voriconazole | Improved |
| Rhizopus spp. | 01 (2.7) | Subcutaneous abdominal swelling | Post renal transplant | Debridement followed by Amphotericin B and Posaconazole | Cured |
Results
A total of 288 pus/tissue non duplicate specimens were received for fungal culture during the study period. Out of these 37 showed growth of fungi. Out of 37 fungi, 24 were yeasts and 13 were mycelial fungi. Direct microscopic examination (KOH mount) of all the specimens with growth of mycelial fungi showed presence of fungal hyphae and those with growth of yeast showed presence of yeast in the specimen. [Table/Fig-2] shows the presence of septate, branching pigmented fungal hyphae in pus specimen from infected ankle cyst of post renal transplant patient. Few of these specimens were also sent for histopathological examination which also corroborated well with KOH mount examination and culture growth. [Table/Fig-3,4] shows the histopathological examination of left ankle infected cyst. The distribution of type of fungal isolates is given in [Table/Fig-1].
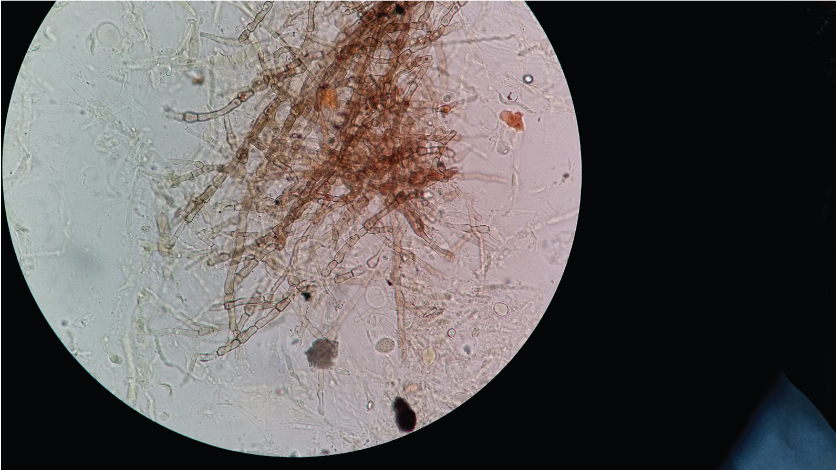
KOH wet mount of pus from infected ankle cyst- shows black pigmented, septate fungal hyphae suggestive of Phaeoid fungi (Magnification 400X).
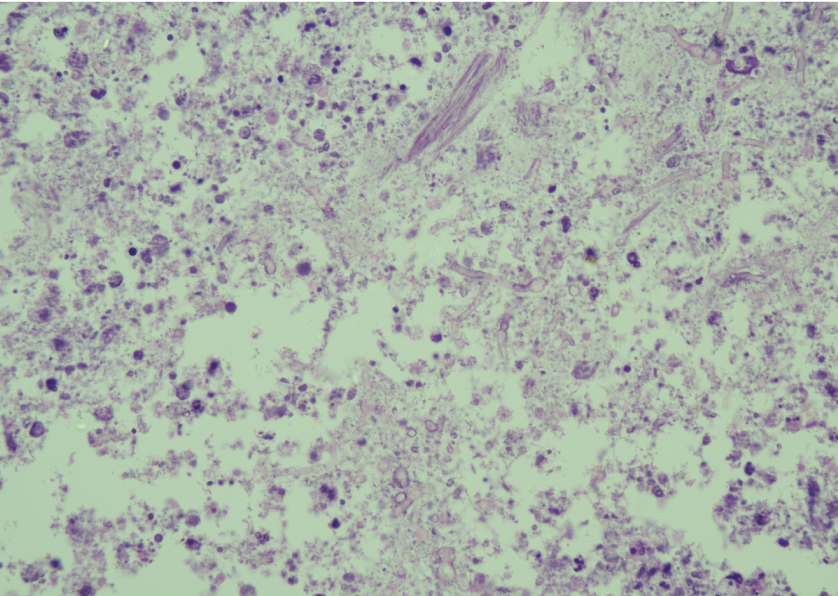
Haematoxylin and Eosin stain- central necrotic area shows fungal hyphae and spore like structure enmeshed with necrotic cell debris (Magnification 400X).
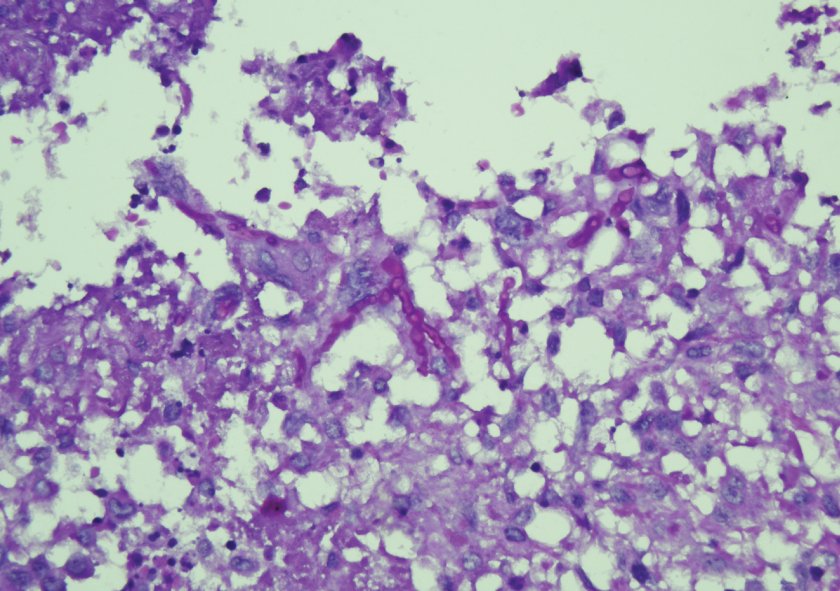
PAS stain- Fungal structures ingested by multinucleated giant cells highlighted on PAS stain (Magnification 400X).
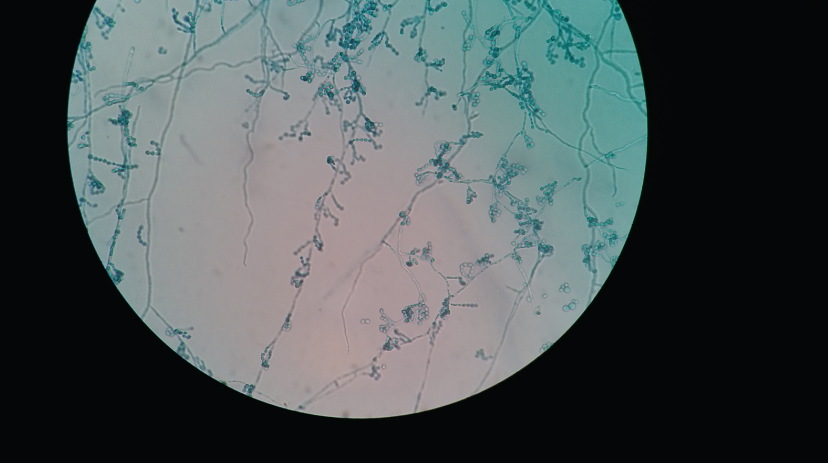
The yeast identification results by Vitek-2 and/or Vitek-MS correlated with conventional methods like germ tube test, Chrome agar and cornmeal agar for the common Candida species like C. albicans, C.tropicalis and C. glabrata. Germ tube test was positive in C. albicans and negative in other species. On Chrome agar (Hicrome Candida differential agar, HiMedia, Mumbai, India), C. albicans showed light green coloured smooth colonies, C. tropicalis showed blue coloured raised colonies and C. glabrata showed cream coloured smooth colonies. On corn meal agar, C. albicans showed pseudohyphae, clusters of blastoconidia and chlamydospores. However, C. glabrata did not show pseudohyphae formation and compactly arranged small yeast cells were seen. C. tropicalis showed presence of single or small cluster of blastoconidia along pseudohyphae. The identification of mycelial fungi was done by LPCB mount of slide culture. [Table/Fig-5,6] shows Exophiala sp. and Cladophialophora sp. on slide culture.
LPCB mount of slide culture-Exophiala sp.- Conidia are one-celled, subhyaline, smooth, thin-walled, sub-globose to ellipsoidal, arising laterally or on tips and and aggregating in clusters. Conidiophores are showing brown pigmented walls (Magnification 400X).
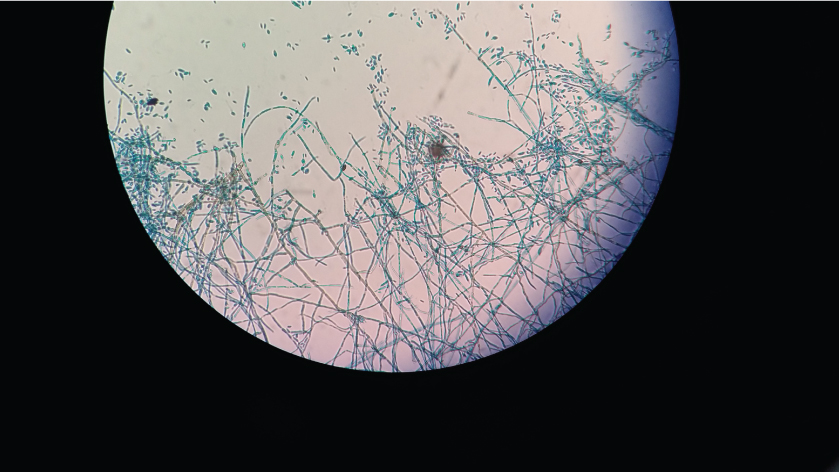
LPCB mount of slide culture-Cladophialophora sp.-Conidia are formed in long, sparsely branched, chains from undifferentiated conidiophores. Conidia are one-celled, pale brown, smooth-walled, ellipsoid to oblong-ellipsoid (Magnification 400X).
The age range of the patient population showing growth of fungi varied from 12 years to 76 years and the median age was 44 years. Out of 37 patients 33 were males and four were females; 12 were immunocompromised (Post transplant and/or on immunosuppressant); 22 had history of surgery (including eight transplant surgeries) and sixteen were diabetic. Out of 37 patients, 18 patients had leucocytes within normal limits, 17 had leucocytosis and two had leucopenia. The distribution of fungal isolates with respect to the site of the specimen, clinical diagnosis, treatment and outcome is depicted in [Table/Fig-1]. The MIC values for various Candida species is depicted in [Table/Fig-7].
Minimum inhibitory concentration (MIC Values) of various Candida species.
| Antifungal drug | Candida albicans(09) | Candida glabrata(08) | Candida haemulonii/auris(03) | Candida tropicalis(03) |
|---|
| M.I.C Values (μg/mL) range |
| Amphotericin B | 0.5-1 | 0.5-1 | ≥8 | 0.5-1 |
| 5 Flucytosine | ≤1 | ≤1 | ≤1 | ≤1 |
| Fluconazole | ≤1 | 4-≥64 | ≥16-64 | ≤1 |
| Voriconazole | ≤0.12 | ≤0.12-1 | ≤0.12 | ≤0.12 |
| Caspofungin | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 |
| Micafungin | ≤0.06-0.12 | ≤0.06-0.12 | 0.12 | ≤0.06 |
Discussion
The frequency and occurrence of candidiasis has been well described [5,6]. More than 80% of high-risk patients in critical care units and those who are neutropenic may be colonized with Candida species, and superficial mucosal and cutaneous infections are common [7,8]. These non-invasive infections can be effectively treated with improved skin care and a topical antifungal agent or with a short course systemic azole antibiotic (e.g., fluconazole). In our study, out of 24 yeast isolates, C. albicans was isolated maximally (37.5%), followed by C. glabrata (33.3%), C. tropicalis (12.5%), C. haemulonii/auris (12.5%) and C. pulcherimma (4.1%). Few of these isolates were present as colonizer and were not necessarily associated with the disease condition. So, the treatment in such cases was started only after clinical correlation especially in view of underlying disease and immune status of the patient. Amongst seven immunosupressed/immunocompromised patients five (71.4%) showed growth of non albicans Candida. However, amongst 17 immunocompetent patients, 07 (41.1%) showed growth of C. albicans. So, the present study shows the predominance of non albicans Candida amongst immunocompromised patients in comparison to immunocompetent patients. It was seen the epidemiology of fungal infections in pus/tissue specimens only. In other studies, where the authors have studied the epidemiology of yeast isolates in all the specimens, the isolation of non albicans Candida have been found to be more than C.albicans.
In our study, amongst non albicans Candida the isolation of C.glabrata was found to be the maximum. Out of eight C.glabrata isolates, six were isolated from abdominal/intraabdominal abscess/tissue. Candida glabrata was also isolated from a case of pancreatic necrosis. Pancreatic infections usually occur due to enteric bacteria. Amongst yeast, the isolation of C.glabrata is rare; C.albicans being reported maximally [9,10]. Chakrabarti A et al., noted Candida tropicalis to be the most common fungal species grown from the samples collected from the pancreatic tissues of 335 patients with acute pancreatitis [11]. For all the isolates of C.glabrata the MIC value for Fluconazole was found to be ≥4 μg/mL. All the isolates of C. glabrata were found to be susceptible to Amphotericin B, 5 Flucytosine, Voriconazole, Caspofungin and Micafungin.
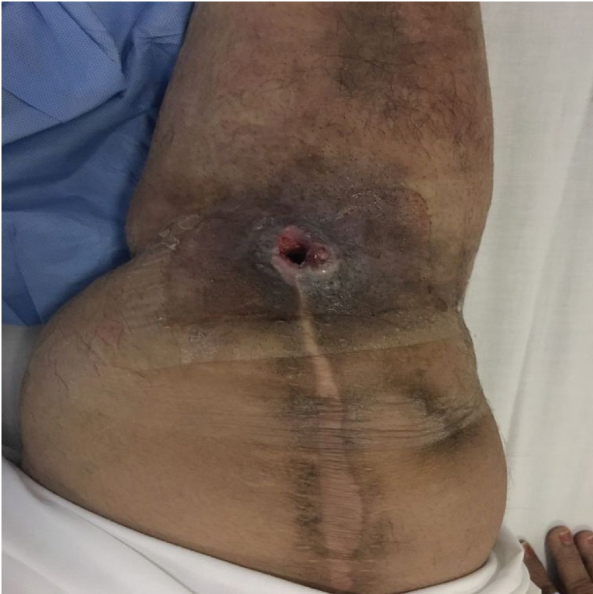
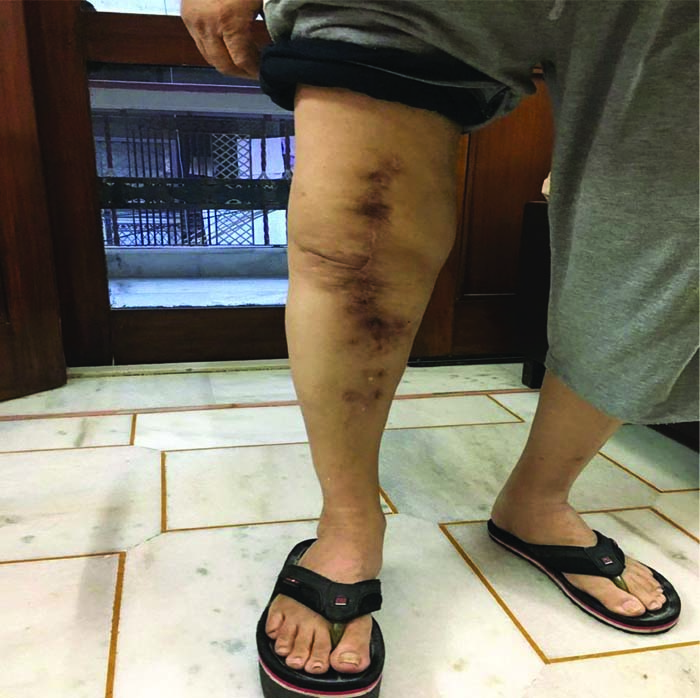
C.haemulonii complex/auris is now emerging as an invasive fungal pathogen especially amongst immunocompromised patients. C. haemulonii complex consists of three genotypically distinguishable species i.e., C. haemulonii, Candida duobushaemulonii and C. haemulonii var. vulnera. There are two other species which are related to C. haemulonii complex and can be misidentified as C. haemulonii. These species are C. auris and C.pseudohaemulonii [12]. C.haemulonii has been reported to be resistant with Amphotericin B and Fluconazole [13,14]. So, it is pertinent to identify these species because antifungal resistance is a great concern with this species. In this study all the three isolates of C. haemulonii/auris were from patients following surgery. In the present study we have also found that the isolates were resistant to amphotericin B and Fluconazole and sensitive to 5-Flucytosine, Voriconazole and Caspofungin. There are reports of misidentification of Candida haemulonii by Vitek-2 YST cards [15]. It has been seen that Vitek-2 system identifies C.auris, C. haemulonii and C. pseudohaemulonii as C. haemulonii [16]. The sequence analysis of the Internal Transcribed Spacer (ITS) region and the D1/D2 domain of the large subunit ribosomal RNA gene identify these isolates as C. auris. As mentioned in [Table/Fig-1], a case of Total Knee Replacement (TKR) is presenting till date with recurrent C. haemulonii/auris infection. The patient has taken several course of Voriconazole with intermittent response. Patient was a long standing diabetic with occasional periods of poor control. Patient also underwent revision TKR followed by Voriconazole for three months. [Table/Fig-8] shows the discharging wound Post TKR and [Table/Fig-9] shows healed wound after revision TKR. In literature, post surgical osteomyelitis by Candida species have been seen but orthopaedic infections due to Candida haemulonii/auris are not reported. This can be due to misidentification or non-identification [17].
Sinus formation at the surgical site (Total knee replacement)- caused by Candida haemulonii/auris (a) 3 incisions; (b) Middle incision showing dissection of Extensor Pollicis Longus tendon (EPL) and Dynamic Compression Plate (DCP); (C) Plate with screws in lateral view
Healed sinus site after revision Total knee replacement followed by oral Voriconazole for three months
In the present study, Candida tropicalis was isolated from hepatic abscess in a 36-year-old male patient who was a case of Gall stone disease with choledocholithiasis. Most of the cases of hepatic abscess have been reported in patients with haematological malignancies and the most common species have been found to be C. albicans [18]. However, Chen CY et al., reported the maximum isolation of Candida tropicalis in cases of hepatosplenic abscess in patients with acute leukaemia [19]. This patient was found to be immunocmpetent and non-neutropenic. Like our case, Menachery J et al., reported liver abscess in a 30-year-old immunocompetent male patient due to C. albicans [18]. The other two isolates of C. tropicalis were isolated from open wound. All the Candida tropicalis isolates in our study were found to be susceptible to all the drugs tested.
Candida pulcherrima was isolated from ulcers on leg in a patient of peripheral vascular disease. This species of Candida has earlier been reported to be involved in nail infections but now there are reports of fungaemia caused by this fungi [20,21].
For the identification of the yeasts, in comparison to conventional techniques, we found use of MALDI-TOF Vitek MS to be time saving, less labour intensive, cost-effective and accurate. The time required for the identification of the yeasts is at least 48 hours using conventional methods while by using MALDI-TOF-MS, the identification of the yeasts can be done in 10 minutes which is very helpful in the initiation of the appropriate antifungal therapy because there are certain species which are inherently resistant to certain antifungal drugs and few species usually show less susceptibility to particular antifungal drugs. MALDI-TOF-MS was also found to be very cost-effective and less labour intensive because the preparation and inoculation of a battery of biochemical tests are not required for the identification. A limited number of reagents and consumables (Target slide, matrix, formic acid, pipette tips, disposable loop or tooth pick) are required for the identification by MALDI-TOF-MS. Moreover, the interpretation of the results is also more specific and accurate and removes any confounding error.
Although, the isolation of Phaeoid fungi has been reported from both immunocompromised and immunocompetent patients but they are increasingly becoming opportunistic pathogens. In our study, all the five Phaeohyphomycetes (Dematiaceous fungi) were isolated from immunocompromised i.e., post-transplant patients. Out of five, two were isolated from brain abscess (cerebral phaeohyphomycosis) and three were isolated from lower limb lesions (subcutaneous phaeohyphomycosis). These fungi are generally found in soil or associated with plants and distributed worldwide. Exposure is thought to be from inhalation or minor trauma. Central nervous system infections can be due to haematogenous spread from an initial, presumably subclinical pulmonary focus. These cases were managed primarily surgically with wide excision and/or debridement of wound. After fungal culture report, Itraconazole was administered in most of the cases.
Aspergillus spp. was isolated mainly from cases of paranasal sinusitis. Aspergillus related sinus syndromes include allergic sinusitis, sinus aspergilloma, chronic granulomatous sinusitis, chronic invasive sinusitis and acute invasive sinusitis [22]. In the present study the patients of paranasal sinusitis were categorised as cases of allergic rhinosinusitis or nasal polyposis. These patients were immunocompetent and showed allergic manifestations like allergic rhinitis and/or asthma. All the cases of Aspergillus rhinosinusitis/polyposis were caused by A. flavus. Prateek S et al., also reported most common isolation of A. flavus amongst cases of both fungal rhinosinusitis and allergic fungal rhinosinusitis [23]. These patients were treated mainly surgically with or without antifungal therapy with good clinical response. Telmesani LM found 12.1% prevalence of Allergic Fungal Sinusitis (AFS) among patients with nasal polyps. They demonstrated Aspergillus sp. in 08 out of 11 cases of AFS [24].
In the present study, one case of cutaneous zygomycosis due to Rhizopus sp. was seen in post renal transplant patient. Bojdy A et al., also reported a case of cutaneous zygomycosis in renal transplant recipient [25].
All the patients in the study had history of prior antibiotic intake for one or other reasons. In contradiction to leucopenia, many of the patients in our study had leucocytosis at the time of isolation of fungi. Fungal infections in 16 patients were associated with history of previous surgery. Majority of these surgeries were performed outside the hospital for one or another reasons like wound repair in case of road traffic accidents, abdominal surgeries etc. Diabetes was seen in 16 (43.2%) patients with fungal infections.
Limitation
The limitation of the study was that, we cannot exactly correlate poor outcome of the patient to the fungal infection or underlying co-morbid condition in immunocompromised patients. The prospective study on this concept especially with emphasis on long term follow-up will help in the optimum management of the patient.
Conclusion
In the present study, we have compiled all the clinically significant data of pus and soft tissue infections caused by fungi in our tertiary health care set-up. In literature, the studies pertaining to fungal isolates specifically from pus and tissue are very scarce. We have important to have such type of data in a clinical set-up because epidemiological profile of fungal infections varies with the type of specimen and patient population.
A variety of fungi may cause pus and soft tissue infections in both immunocompromised and immunocompetent individuals. So, it is important to diagnose fungal infections, identify the fungal species and know its antifungal susceptibility pattern to avoid unnecessary usage of antibiotics and appropriate antifungal therapy. A clinico-mycological awareness and correlation is required for optimum management.