Multiple primary tumours in a single individual are reported in the literature with incidence which varies from 1% to 20%. Few common combinations are labelled as the syndrome and showed a common genetic association; however, sporadic cases of multiple primaries are relatively rare. Such a combination of Renal Cell Carcinoma (RCC) with pancreatic carcinoma is sparingly being reported in the literature. Here, we present a case of pancreatic carcinoma in a patient who was treated for renal cell carcinoma, 10-years-back. A few cases, which have been published before also could not establish any specific reason for this combination. We recommend considering a possibility of the second primary in any patient with unusual symptoms different from primary lesion and research for genetic association in these patients.
Case Report
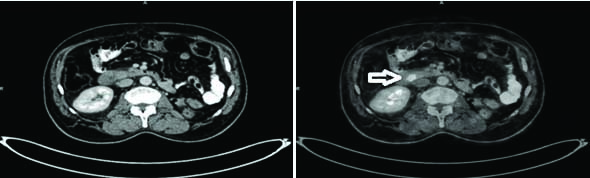
A 65-year-old male presented to the Department of Gastroenterology surgery with a history of left nephrectomy for renal cell carcinoma 10 years ago. Post-nephrectomy he was on continuous follow-up. He received interferon therapy followed by sunitinib for three years for the metastatic lesion in the chest and left renal bed. His metastatic disease was under control with treatment and repeated Positron Emission Tomography (PET) scans showed static disease in both chest and the renal bed. This time he presented with a history of low-grade fever for 10 days, followed by vomiting and generalised itching for two days. His PET CT scan revealed an FDG avid nodular lesion of size 1×1.5 cm in the periampullary region (new finding) and metastatic lesion in the chest and left renal fossa, comparable to the previous scan [Table/Fig-1]. Total leukocyte count was normal, but his liver function test was deranged with Serum bilirubin 1.5 mg/dL, alkaline phosphatase 1084 IU/dL and gamma glutamyl transferase 1042 IU/dL. His CA 19.9 level was 75 IU/dL.
FDG PET scan showing periampullary tumour (arrow).
He underwent an endoscopic biopsy to rule out metastasis from primary renal cell carcinoma and biliary stenting for cholangitis. Histopathology of the endoscopic biopsy revealed poorly differentiated adenocarcinoma, consistent with pancreatic origin. Immunohistochemistry for synaptophysin and chromogranin was negative which ruled out renal cell origin. The patient was physically fit with ECOG score 1, so planned for staging laparoscopy followed by surgery for periampullary growth.
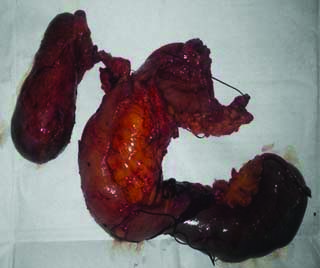
On exploration, the tumour was localised to the periampullary region without any localised spread or evidence of distant metastasis. The hard nodular growth of size 1×1.5×1 cm was present at the pancreatic head, infiltrating duodenum [Table/Fig-2]. Pancreaticoduodenectomy was done and the patient recovered well after surgery. Histopathology of excised specimen revealed poorly differentiated adenocarcinoma of pancreatic-type. Tumour infiltrated the muscularis propria of the duodenum and focally invaded the terminal part of the common bile duct superficially. There was no evidence of nodal metastasis and final pathological stage was T2N0. Postoperatively patient is doing well with a follow-up period of six months.
Resected specimen of pancreaticoduodenectomy (1×1.5×1 cm).
Discussion
Renal Cell Carcinoma (RCC) is the most common malignant tumour of kidney and accounts for approximately 2% of all cancer diagnoses and cancer deaths worldwide, with incidence rates generally higher in developed countries [1]. RCC may present as both sporadic and hereditary forms. Various risk factors described for development of RCC include smoking, hypertension, obesity, end-stage renal disease, occupational exposures (aromatic hydrocarbons, asbestos, cadmium, and chemical and rubber industries) and several genetic syndromes including von Hippel-Lindau syndrome [2].
RCC is prone to metastasis and according to SEER database report, up to 37% patients present with distant metastasis and in 20% of patients, who initially present with the localised disease develop metastasis in future. Common sites of metastasis include lung (75%) and lymph nodes (36%) followed by the bones (20%) and liver (18%). Few cases of metastasis to the gastrointestinal system have been reported [3].
RCC is also having shown association with the development of several second primary malignancies due to immunosuppressive effect and common genetic predisposition. These include non-hodgkin’s lymphoma, multiple myeloma, chronic lymphatic leukaemia, melanoma and cancers of the bladder, prostate, breast, rectum, and lung with an incidence that ranges from 5% to 27% [4,5].
Pancreatic, biliary or duodenal malignancies are infrequently reported in association with RCC. Rabbani F et al., analysed the data of Memorial Sloan Kettering Cancer Center, New York, noticed multiple primary malignancies in 27% of 763 RCC patients, commonly prostate, bladder and colorectal cancer and non-hodgkin’s lymphoma; however, no periampullary tumours [6].
Pancreatic Ductal Adenocarcinoma (PDAC) comprises 2-3% of all cancers in adults and highly malignant in nature. These patients usually present late due to vague symptoms and only 10-20% of patients are amenable to cure by surgical resection. Although, exact factors for the development of PDAC are not known, few risk factors commonly quoted are age, smoking, obesity and chronic pancreatitis. Five percent of all cases of PDAC are associated with genetic disorders including Lynch syndrome, BRCA1 and BRCA2 mutation and familial atypical malignant melanoma [7].
As reported by Kamisawa T et al., PDAC is seen with high incidence (1%-20%) in patients with other gastrointestinal malignancies, especially gastric carcinoma [8]. Gerdes B et al., analysed 69 patients with multiple malignancies and among them, 13 had PDAC with second primary malignancies; however, none of them was found to have RCC [9].
Sasaki E et al., reported a case of double cancer involving pancreas and kidney in 1969 for the first time [10]. Thereafter not many cases have been reported in the literature. Alexakis N et al., reported two cases of synchronous RCC and PDCA in the year 2003 [11].
Kantor AF et al., published an analysis of the Connecticut tumour registry for the period 1935-1982. They revealed that 19% of 4176 patients with renal cell cancer developed a second primary malignancy. They found six cases of pancreatic cancer in association with RCC [12].
Largest series on this aspect was published by Müller SA et al., in 2012. They retrospectively analysed 1178 patients with pancreatic tumour and 518 patients with RCC who underwent surgery between 2001 and 2008. They found 12 cases of PDAC and four cases of IPMN in patients with RCC. Among 12 cases of PDAC, three cases had synchronous presentation, while nine cases had the metachronous presentation in the follow-up (12-205 months) [13].
In the present case, the patient was well preserved with metastatic RCC. Since, he was well controlled with sunitinib for more than three years and he had ECOG 1 status, he was considered for surgery. He is now continued on sunitinib in the adjuvant setting.
Due to the increased survival rate of cancer patients and improved diagnostic studies, especially radiologic imaging, there has been an increasing trend in the detection of synchronous or metachronous second malignancy. Although, the mechanism behind the development of multiple primary cancers is not well understood, several factors can be considered for such a predisposition. These factors may include environmental (e.g., smoking, alcohol, obesity, occupation), genetic, and effect of chemotherapy or radiotherapy for previous cancer. Studies done for the genetic predisposition of cancer revealed that microsatellite instability is more common in multiple primary malignancies than sporadic cancers [14].
Smoking is a common risk factor for both RCC and PDAC and has approximately two folds increased the risk of both cancers. Other predisposing factors may be genetic factors, which are still not clear. Von Hippel-Lindau (VHL) disease is a rare genetic disorder characterised by multiple cystic and solid lesions in multiple organ systems that have the potential for malignant change. This disease is characterised by retinal and brain hemangioblastoma, multiple cysts in the kidney and pancreas. Renal cysts have increased the risk for development of RCC. The pancreatic lesion may progress to a cystadenoma or a neuroendocrine tumour; however, no incidence of PDAC has been reported in these patients [15].
Nevertheless, we believe that there could be a new association between these two primary tumours. Further analytic epidemiological studies, including evaluation of gene-environment interactions, are required to specifically identify reasons for double pancreatic-kidney tumours.
Conclusion
As number of patients presenting with multiple malignancies are increasing everyone should be aware of this. The presentation could be either synchronous or metachronous. Prognosis of these patients depends on the primary malignancy itself. This article is presented here to create awareness among medical faculty about the multiplicity of tumours with RCC.
[1]. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] 2013 Lyon, FranceInternational Agency for Research on CancerAvailable from: http://globocan.iarc.fr, accessed on day/month/year [Google Scholar]
[2]. Chow WH, Dong LM, Devesa SS, Epidemiology and risk factors for kidney cancerNat Rev Urol 2010 7(5):245-57.10.1038/nrurol.2010.4620448658 [Google Scholar] [CrossRef] [PubMed]
[3]. Sadler GJ, Anderson MR, Moss MS, Wilson PG, Metastases from renal cell carcinoma presenting as gastrointestinal bleeding: two case reports and a review of the literatureBMC Gastroenterol 2007 7:410.1186/1471-230X-7-417266757 [Google Scholar] [CrossRef] [PubMed]
[4]. Koyama K, Furukawa Y, Tanaka H, Multiple primary malignant neoplasms in urologic patientsScand J Urol Nephrol 1995 29:483-90.10.3109/003655995091800318719367 [Google Scholar] [CrossRef] [PubMed]
[5]. Liu H, Hemminki K, Sundquist J, Renal cell carcinoma as first and second primary cancer: etiological clues from the Swedish family-cancer databaseJ Urol 2011 185(6):2045-49.10.1016/j.juro.2011.02.00121496838 [Google Scholar] [CrossRef] [PubMed]
[6]. Rabbani F, Reuter V, Katz J, Russo P, Second primary malignancies associated with renal cell carcinoma. Influence of histologic typeUrology 2000 56:399-403.10.1016/S0090-4295(00)00682-8 [Google Scholar] [CrossRef]
[7]. Lowenfels AB, Maisonneuve P, Epidemiology and risk factors for pancreatic cancerBest Pract Res Clin Gastroenterol 2006 20:197-209.10.1016/j.bpg.2005.10.00116549324 [Google Scholar] [CrossRef] [PubMed]
[8]. Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A, The incidence of pancreatic and extrapancreatic cancer in Japenese patients with chronic pancreatitisHepatogastroenterology 2007 54:1579-81. [Google Scholar]
[9]. Gerdes B, Ziegler A, Ramaswamy A, Wild A, Langer P, Bartsch DK, Multiple primaries in pancreatic cancer patients: indicator of a genetic predisposition?Int J Epidemiol 2000 29(6):999-1003.10.1093/ije/29.6.99911101540 [Google Scholar] [CrossRef] [PubMed]
[10]. Sasaki E, Kushida S, Okinaka T, Case report of double cancer of the pancreas and the kidney with polyposis of the large intestineGan No Rinsho 1969 15(2):203-06. [Google Scholar]
[11]. Alexakis N, Bosonnet L, Connor S, Ellis I, Sutton R, Campbell F, Double resection for patients with pancreatic cancer and a second primary renal cell cancerDig Surg 2003 20:428-32.10.1159/00007271112900534 [Google Scholar] [CrossRef] [PubMed]
[12]. Kantor AF, McLaughlin JK, Curtis RE, Flannery JT, Fraumeni JF, Risk of second malignancy after cancers of the renal parenchyma, renal pelvis and ureterCancer 1986 58(5):1158-61.10.1002/1097-0142(19860901)58:5<1158::AID-CNCR2820580530>3.0.CO;2-V [Google Scholar] [CrossRef]
[13]. Müller SA, Pahernik S, Hinz U, Martin DJ, Moritz N, Wente MN, Renal tumours and second primary pancreatic tumours: a relationship with clinical impact?Patient Saf Surg 2012 6:1810.1186/1754-9493-6-1822873581 [Google Scholar] [CrossRef] [PubMed]
[14]. Beisland C, Talleraas O, Bakke A, Norstein J, Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort studyBJU Int 2006 97:698-702.10.1111/j.1464-410X.2006.06004.x16536756 [Google Scholar] [CrossRef] [PubMed]
[15]. Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel-LindauGastroenterology 2000 119:1087-95.10.1053/gast.2000.1814311040195 [Google Scholar] [CrossRef] [PubMed]