The Prevalence and Profile of Mycobacterial Infections in Liver Diseases from Tertiary Care Hepatobiliary Centre
Pratibha Kale1, Vikas Khillan2, Pradheer Gupta3, Shiv Kumar Sarin4
1 Assistant Professor, Department of Clinical Microbiology, Institiute of Liver and Biliary Sciences, New Delhi, India.
2 Additional Professor, Department of Clinical Microbiology, Institiute of Liver and Biliary Sciences, New Delhi, India.
3 Senior Resident, Department of Clinical Microbiology, Institiute of Liver and Biliary Sciences, New Delhi, India.
4 Professor, Department of Hepatology, Institiute of Liver and Biliary Sciences, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Vikas Khillan, Additional Professor, Department of Clinical Microbiology, Institute of Liver and Biliary Sciences, Delhi-110070, India.
E-mail: vkhillanilbs@gmail.com
Introduction
There is paucity of data regarding mycobacterial infections in liver diseases. Guidelines do not exist for diagnosis, monitoring of patients and modification of the treatment.
Aim
Our aim was to elucidate demographic characteristics, profile of mycobacterial infection and comparison of diagnostic methods in liver diseases.
Materials and Methods
We studied liver disease patients from January 2012 to December 2016, screened for Tuberculosis (TB) if having fever, cough for >2 weeks, haemoptysis, unexplained weight loss, increasing ascites, unresponsive to diuretics, unexplained bowel symptoms, radiological lesions and past or family history of TB. TB diagnosed if there is: (i) histological caseating granulomas; (ii) smear Acid Fast Bacilli; (AFB) positivity; (iii) growth on Mycobacterial Growth Indicator Tube (MGIT) culture; or (iv) positive quantitative polymerase chain reaction for Mycobacterium tuberculosis (MTB qPCR). Mycobacteria other than tuberculosis (MOTT) were identified by negative MPT64 assay.
Results
Of the 118/816 positive samples, 31/260 (11.92%) were pulmonary and 87/556 (15.65%) were extra-pulmonary TB (EPTB). There was a male preponderance (66.1%), median age 53 years in pulmonary and 37 years in EPTB. Thirty two samples (27.11%) were smear positive, low in EPTB 13/87 (14.9%) as compared to 19/31 (61.2%) pulmonary. MGIT was positive in 108/118 (91.52%) and 97/118 (82.2%) were MTB qPCR positive. MTB was isolated from all pulmonary samples and 72/87 (82.75%) of EPTB. MOTT was identified in 15/118(12.71%). Sensitivity and specificity of MTB qPCR was 90.3% and 100% respectively in pulmonary and 76.6% and 97.9% respectively in EPTB.
Conclusion
There is predominance of smear negative, EPTB and MOTT in liver diseases. MGIT culture and TB PCR have additive advantage over either test alone. MOTT should be ruled out in all cases as treatment varies. High index of suspicion and good screening methods are needed to identify TB and MOTT owing to similar presentation and anti-tubercular drug toxicity issues.
Diagnosis, Extra-pulmonary tuberculosis, Mycobacteria other than tuberculosis
Introduction
Tuberculosis is a major health crisis in developing countries like India. TB is the foremost killer of about 1,400 every day and approximately 480,000 Indians every year [1]. There is an issue of missing cases, as most cases are undiagnosed or inadequately diagnosed and treated. Pulmonary TB is amenable to diagnosis due to overt signs and symptoms and increasing awareness with rapid medical advice. However, EPTB often goes undiagnosed, owing to the associated disease symptoms masking the TB or paucibacillary disease making it difficult to diagnose. TB has a complex relationship with liver diseases, as the diseases may affect liver in disseminated cases with miliary disease and can be reactivated in immunosuppressive conditions or could affect the liver with toxic anti-tubercular therapy. There is paucity of data regarding mycobacterial infections in hepatobiliary disease patients and guidelines do not exist regarding diagnosis, monitoring of such patients or modification of the anti-tubercular regimen. Characteristics of TB in patients with cirrhosis, alcoholic liver disease, chronic liver failure, post hepatitis liver disease, pancreatitis etc. in terms of clinical characteristics, treatment responses and adverse effects are largely unknown. We planned this study to elucidate demographic characteristics of TB, pattern and prevalence of infection in hepatobiliary diseases.
Materials and Methods
We analysed liver disease patients admitted to our tertiary care centre from January 2012 to December 2016 in this retrospective study. Patients were screened for TB only if patients presented with: fever, cough for >2 weeks, weight loss, haemoptysis, and unresponsive ascites, arcane bowel symptoms (constipation, diarrhoea, or subacute intestinal obstruction), and radiological features reminiscent of TB and past or family history of TB. Samples received were alienated as pulmonary (sputum, bronchoalveolar lavage) and extrapulmonary (lymphnode samples, tissue biopsy, urine, and body fluids as ascitic fluid, pleural fluid etc.,). The samples were subjected to microscopy with Zeihl Nelsen staining, TB was diagnosed based on one of the following: (i) histological evidence of caseating granulomas; (ii) smear positivity for AFB; (iii) growth of Mycobacteria on MGIT culture (BD Microbiology Systems, Cockeysville, MD); or (iv) positive PCR for Mycobacterium tuberculosis in tissues (MTB qPCR) (Cobas TaqMan MTB assay). Mycobacteria other than tuberculosis (MOTT) were identified by negative MPT64 immunochromatography assay (SD Bioline) or MGIT culture positive and MTB PCR negative samples. Statistical data analyses were performed using SPSS Statistics 19 (SPSS Inc., Chicago, IL, USA). Sensitivity and specificity of the tests were calculated using culture results as gold standard. The study was approved by Institute ethical committee.
Results
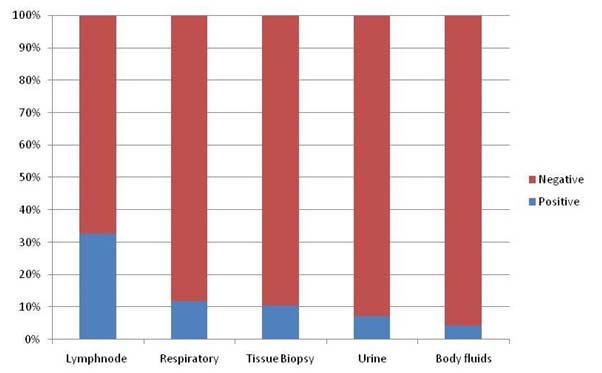
A total of 816 samples were processed for mycobacterial identification. These included 260 pulmonary and 556 extra-pulmonary samples. A total of 118 were positive by MGIT culture or PCR inclusive of pulmonary 31/260 (11.92%) and extra pulmonary 87/556 (15.65%) samples. The underlying liver disease was predominantly cirrhosis 72/118 (61.01%), alcoholic liver disease 25/118 (21.1%), post liver transplant 6/118 (5.08%), non-alcoholic steatohepatitis 3/118 (2.54%), liver malignancy or secondaries 2/118 (1.69%), hydatid disease of liver 1/118 (0.84%), viral hepatitis 2/118 (1.69%) and pancreatic malignancy 2/118 (1.69%), pancreatitis 2/118 (1.69%), an ulcerative colitis 3/118 (2.54%) amongst other causes. The system wise distribution of positive samples as respiratory (11.92%), lymphnodes (32.67%), tissue biopsy (10.41%), urine (7.4%) and body fluids (4.44%) are given in [Table/Fig-1]. The demographic profile revealed 78/118 (66.1%) male and 40/118 (33.8%) female patients with positive results. The distribution of age and sex in pulmonary and extrapulmonary diseases is depicted in [Table/Fig-2]. Smear microscopy was positive in 32/118 (27.11%) which was higher in pulmonary 19/31 (61.2%) than extrapulmonary samples 13/87 (14.9%). The samples exclusively positive on MGIT culture system were 108/118 (91.52%) and those positive on MTB qPCR were 97/118 (82.2%). Mycobacterium tuberculosis was identified in 103/118 (87.28%) and MOTT was detected in 15/118 (12.71%) samples. The pulmonary and extrapulmonary distribution of these species are given in [Table/Fig-2]. The sensitivity of MTB qPCR when compared to MGIT culture as gold standard was 90.3% (95% CI, 73-97%) for pulmonary disease and 76.6% (95% CI,65.3-85.2) for extrapulmonary disease [Table/Fig-3]. The specificity of MTB qPCR when compared to MGIT culture was 100% (95% CI, 97.9-100%) in pulmonary samples and 97.9% (95% CI, 96-98.9) in extra-pulmonary samples [Table/Fig-3]. In our study the positive predictive value of MTB qPCR in pulmonary disease was 100% while in non-pulmonary disease was 85.5% (95% CI, 74.4- 92.4%). The negative predictive value was 98.7% (95% CI, 95.9-99.6%) and 96.3% (95% CI, 94.1- 97.7%) in pulmonary and extrapulmonary samples respectively [Table/Fig-3].
Systemwise distribution of positive samples.
Characteristic of pulmonary and extrapulmonary disease.
| Pulmonary disease | Extrapulmonary Disease | Total |
|---|
| Age (years, median) | 53 | 37 | |
| Male | 26 | 52 | 78 |
| Female | 5 | 35 | 40 |
| Positive | 31 | 87 | 118 |
| MTB n(%) | 31 (100) | 72 (82.75) | 103 |
| MOTT n(%) | 0 | 15 (12.71% and 17.24% in extrapulmonary samples) | 15 |
| Smear positive n(%) | 19 (61.2%) | 13 (14.9%) | 32 (27.11%) |
MTB-Mycobacterium Tuberculosis, MOTT-Mycobacteria Other Than Tuberculosis
Comparison of culture and MTB qPCR in pulmonary and extrapulmonary disease.
| Pulmonary disease | Extra-pulmonary disease |
|---|
| Culture | MTB qPCR | Culture | MTB qPCR |
|---|
| Positive | 31 | 28 | 77 | 69 |
| Negative | 229 | 232 | 479 | 487 |
| Sensitivity (95%CI) | Gold standard | 90.3% (73-97) | Gold standard | 76.6% (65.3-85.2) |
| Specificity (95% CI) | 100 (97.9-100) | 97.9% (96-98.9) |
| Positive predictive value (95% CI) | 100% (84.9-100) | 85.5% (74.4- 92.4) |
| Negative predictive value (95% CI) | 98.7% (96.3-99.6) | 96.3% (94.1- 97.7) |
Culture – MGIT culture, MTB qPCR- MTB quantitative real time polymerase chain reaction, CI- confidence interval
Discussion
TB is still epidemic in India carrying more than 20% of the global TB burden, despite of efforts taken to minimise it [1]. The stigma linked with TB in society has harsh consequences on infected individuals. Although, the treatment approach using drugs in combination is successful in treating TB, it may lead to increased side effects with toxicity.
Hepatotoxicity is common with the use of anti-tubercular drugs and often exasperated by the cumulative toxicity of such drug combinations. TB and the liver have complex relationship. The direct involvement of liver by the disease itself is common but marked impairment of liver functions is seldom seen [2]. Chronic liver disease patients are prone to develop TB posing management problems. The underlying disease process may be aggravated as potent anti-tubercular drugs are hepatotoxic. Thus, we need to modify the drug regimens to avert further hepatic insult. Moreover, chronic liver disease is one of the most important risk factor for the development of ATT induced hepatitis also [3]. The absorption and distribution of drugs metabolised in the liver such as rifampicin and isoniazid can be altered due to hepatic dysfunction [4]. Chronic liver disease, being an immunocompromised state augments the risk of activation of latent TB [3,4]. Conversely, hepatic involvement by TB can lead to deterioration of underlying liver injury in liver disease patients [4]. EPTB is difficult to diagnose with concomitant liver disease. The characteristic features of ascitic fluid in peritoneal TB is raised LDH (>90 U/L), increased protein (>2.5 g/L) and low SAAG (<1.1 g/L) levels. However, the diagnosis of tubercular ascites may be confounded by a lower protein and higher SAAG levels in the ascitic fluid with coexistent chronic liver disease [5]. The fluctuations in the liver function profile owing to a pre-existing liver disease causes difficulty in biochemical monitoring the degree of drug induced hepatic injury [6,7].
Pulmonary TB accounts for approximately 80-85% of all cases in the general population [8]. However, in patients with cirrhosis, EPTB is more common. In a Korean study, 31% cirrhotics had EPTB as compared to 12% in the non-cirrhotic control group with predominance of peritoneal TB [9]. Our study reiterates this finding with 15.65% EPTB cases as compared to 11.92% pulmonary cases. Liver cirrhosis as a risk factor for EPTB has been suggested in a previous study [10-12]. Immunopathogenesis of TB in such clinical conditions is still ambiguous. It has been proposed that although most of the host defence mechanisms, particularly the reticuloendothelial system clearance capacities, are dampened in patients with cirrhosis, nonetheless it is yet not clear how this immune dysfunction results in patients being more prone to extrapulmonary TB than pulmonary TB [5,13].
A definitive diagnosis of TB by culturing Mycobacterium tuberculosis organisms from samples obtained from the patient. However, EPTB diagnosis remains challenging as clinical samples obtained from relatively inaccessible extrapulmonary sites may be paucibacillary, which decreases the sensitivity of diagnostic assays. Since the conventional smear microscopy has a low sensitivity with a range of 0%–40%, negative results cannot exclude the presence of TB [14]. Our study shows overall sensitivity of staining as 27.12%, thus, reliance only on microscopy could lead to wrong diagnosis in more than 2/3rd of cases. Smear positivity was much lower in extrapulmonary disease (14.9%) than the pulmonary disease (61.2%). The reported yields of mycobacterial culture vary from 30% up to 80%, but it usually takes 2–6 weeks to receive the results, which is too slow to help treatment decisions [14]. In this study we had culture positivity in (89.83%) and majority of the cultures were positive by third week of incubation. Our culture results were promising as the processing involved stringent decontamination of samples.
Diagnosis of EPTB from tissue samples is usually made by histopathological examination that depends on the presence of granulomatous inflammation and caseous necrosis [15-17]. However; histopatholology does not distinguish between EPTB and infections from other granulomatous diseases such as nontubercular mycobacteria, leprosy, sarcoidosis, and systemic lupus erythematosus [16,17].
Molecular techniques yield rapid results with high sensitivity and shown new vigor in TB diagnosis [15]. Real-time PCR is a novel and robust assay primarily used to quantify the nucleic acid molecules in specimens [18,19]. This technique is advantageous owing to shortened turnaround time, aiding quantification of bacterial load and automation of the procedure reducing hands-on time and decreased risk of cross contamination [19,20]. Because of extremely high sensitivity of PCR, false positivity is also common due to the carry over contamination of amplicon, previous infection or asymptomatic EPTB infection at another site [21,22]. The false positivity of PCR in cases without clinical findings challenges the diagnosis in EPTB cases [23]. Several meta-analyses of commercial Nucleic Acid Amplification Tests (NAATs) have documented sensitivities of 90% to 100% and specificities of 71% to 96% in sputum smear positive samples, but significantly lower sensitivities of 22% to 89% and specificities of 97% to 99% in sputum smear-negative samples [24-26]. For diagnosis of EPTB, performance of NAATs generally is unsatisfactory, signifying pooled sensitivity and specificity of 53% and 95%, respectively for serum, and 30% to 76% and 73% to 99%, respectively for pleural TB [24]. In our study the qPCR was positive in 97/118 (82.2%) with higher sensitivity in pulmonary (90.3%, 95% CI 73-97) than EPTB (76.6%, 95%CI 65.3-85.2). Similarly the specificity for pulmonary disease was 100% compared to EPTB 97.9%. Our study revealed an acceptable positive and negative predictive value of the real time PCR assay [Table/Fig-3]. We adopted a policy to process only those samples with appropriate quality and quantity followed by adequate decontamination when required. The sample collection is most important part for diagnosis of EPTB and we have a system of repeated instructions and training for appropriate sample collection, which has helped increased the positivity of tests. It is noteworthy to mention that, PCR and culture, rather than either test alone could be a useful method to accurately identify TB cases.
Several studies have shown that period prevalence of pulmonary MOTT disease has been highest among Japanese, Chinese, and Vietnamese patients (>300/100,000 persons) and lowest among Native Hawaiians and Other Pacific Islanders (50/100,000) [27,28]. In previous reports, persons identified as Asian American/Pacific Islander were at increased risk for pulmonary MOTT disease, independent of geographic area of residence [27,28]. In an Indian study on MOTT in tertiary care centres in north India, the isolation rate was 62/227 (27.4%) in extra pulmonary samples [29]. Various studies from India showed MOTT prevalence of 17.4% from clinical specimens in patients with fibro cavitary disease and 7.4% from various clinical specimens [30]. In our study the incidence of MOTT disease was 12.71%, isolated from extrapulmonary samples only. Hence, it is necessary to suspect non tubercular mycobacteria in extrapulmonary disease especially with an underlying liver disease. This difference in occurrence of MOTT in extrapulmonary disease needs further evaluation. The studies on MOTT in liver diseases are meagre and more data generation in this subject is needed.
Conclusion
Thus, our study gives important insights into the characteristics of mycobacterial infections in liver diseases. Our study demonstrates the predominance of smear negative, EPTB and MOTT in our patients with underlying liver diseases. A combination of MGIT culture and TB PCR has additive advantage over either test alone. A high index of suspicion and good screening methods are needed to identify TB and MOTT in such patients, owing to similar presentation and anti-tubercular drug toxicity issues. Rapid speciation that distinguishes MTB from MOTT is an important prerequisite for the proper management of patients with mycobacterial infections in liver diseases and may be introduced as a required standard into routine laboratory diagnostics.
MTB-Mycobacterium Tuberculosis, MOTT-Mycobacteria Other Than Tuberculosis
Culture – MGIT culture, MTB qPCR- MTB quantitative real time polymerase chain reaction, CI- confidence interval
[1]. Global Tuberculosis Control 2015, WHO, Geneva, 2015 [Google Scholar]
[2]. Essop AR, Posen JA, Hodkinson JH, Segal I, Tuberculosis hepatitis: a clinical review of 96 casesQJ Med 1984 53:465-77. [Google Scholar]
[3]. Rom WN, Garay SM, Tuberculosis 2004 PhiladelphiaLippincott Williams & Wilkins [Google Scholar]
[4]. Kimerling ME, Phillips P, Patterson P, Hall M, Robinson CA, Dunlap NE, Low serum antimycobcterial drug levels in non- HIV infected tuberculosis patientsChest 1998 113:1178-83.10.1378/chest.113.5.11789596291 [Google Scholar] [CrossRef] [PubMed]
[5]. Shakil AO, Korula J, Kanel GC, Murray NG, Reynolds TB, Diagnostic features of tuberculous peritonitis in the absence and presence of chronic liver disease: a case control studyAmer J Med 1996 100:179-85.10.1016/S0002-9343(97)89456-9 [Google Scholar] [CrossRef]
[6]. Bell LN, Chalasani N, Epidemiology of idiosyncratic drug-induced liver injurySeminars in Liver Disease 2009 29:337-47.10.1055/s-0029-124000219826967 [Google Scholar] [CrossRef] [PubMed]
[7]. Teleman MD, Chee CB, Earnest A, Wang YT, Hepatotoxicity of tuberculosis chemotherapy under general programme conditions in SingaporeInt J Tuberc Lung Dis 2002 6:699-705. [Google Scholar]
[8]. Sharma SK, Ryan H, Khaparde S, Sachdeva KS, Singh AD, Mohan A, Index-TB Guidelines: Guidelines on extrapulmonary tuberculosis for IndiaThe Indian Journal of Medical Research 2017 145:448-63. [Google Scholar]
[9]. Cho YZ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Clinical characteristics of tuberculosis in patients with liver cirrhosisRespirology 2007 12:401-05.10.1111/j.1440-1843.2007.01069.x17539845 [Google Scholar] [CrossRef] [PubMed]
[10]. Gonzalez OY, Adams G, Teeter LD, Bui TT, Musser JM, Extra-pulmonary manifestations in a large metropolitan area with a low incidence of tuberculosisInt J Tuberc. Lung Dis 2003 7:1178-85. [Google Scholar]
[11]. Tanaka A, Kato Y, Arai K, Oh-i T, Koga M, Unusual clinical features of cutaneous tuberculosis in an immune compromised patientJ. Dermatol 2002 29:226-31.10.1111/j.1346-8138.2002.tb00254.x12027088 [Google Scholar] [CrossRef] [PubMed]
[12]. Sharma P, Tyagi P, Singla V, Bansal N, Kumar A, Arora A, Clinical and biochemical profile of tuberculosis in patients with liver cirrhosisJournal of Clinical and Experimental Hepatology 2015 5:8-13.10.1016/j.jceh.2015.01.00325941429 [Google Scholar] [CrossRef] [PubMed]
[13]. Vilstrup H, Cirrhosis and bacterial infectionsRom. J. Gastroenterol 2003 12:297-302. [Google Scholar]
[14]. Canadian Thoracic Society and The Public Health Agency of Canada and LicensorsCanadian tuberculosis standards 2013 7th edOttawaPublic Health Agency of Canada [Google Scholar]
[15]. Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Rapid molecular detection of tuberculosis and rifampin resistanceThe New England Journal of Medicine 2010 363(11):1005-15.10.1056/NEJMoa090784720825313 [Google Scholar] [CrossRef] [PubMed]
[16]. Liu KT, Su WJ, Perng RP, Clinical utility of polymerase chain reaction for diagnosis of smear-negative pleural tuberculosisJ Chin Med Assoc 2007 70:148-51.10.1016/S1726-4901(09)70348-X [Google Scholar] [CrossRef]
[17]. Almadi MA, Ghosh S, Aljebreen AM, Differentiating intestinal tuberculosis from Crohn’s disease: a diagnostic challengeAm J Gastroenterol 2009 104:1003-12.10.1038/ajg.2008.16219240705 [Google Scholar] [CrossRef] [PubMed]
[18]. Baba K, Pathak S, Sviland L, Langeland N, Hoosen AA, Asjo B, Real-time quantitative PCR in the diagnosis of tuberculosis in formalin-fixed paraffin-embedded pleural tissue in patients from a high HIV endemic areaDiagn Mol Pathol 2008 17:112-17.10.1097/PDM.0b013e31814ceac318382372 [Google Scholar] [CrossRef] [PubMed]
[19]. Rosso F, Michelon CT, Sperhacke RD, Verza M, Olival L, Conde MB, Evaluation of real-time PCR of patient pleural effusion for diagnosis of tuberculosisBMC Res Notes 2011 4:27910.1186/1756-0500-4-27921819571 [Google Scholar] [CrossRef] [PubMed]
[20]. Kalantri Y, Hemvani N, Chitnis DS, Evaluation of real-time polymerase chain reaction, interferon-gamma, adenosine deaminase, and immunoglobulin A for the efficient diagnosis of pleural tuberculosisInt J Infect Dis 2011 15:e226-31.10.1016/j.ijid.2010.11.01121227729 [Google Scholar] [CrossRef] [PubMed]
[21]. Honore-Bouakline S, Vincensini JP, Giacuzzo V, Lagrange PH, Herrmann JL, Rapid diagnosis of extrapulmonary tuberculosis by PCR: impact of sample preparation and DNA extractionJ Clin Microbiol 2003 41:2323-29.10.1128/JCM.41.6.2323-2329.200312791844 [Google Scholar] [CrossRef] [PubMed]
[22]. Chakravorty S, Sen MK, Tyagi JS, Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technologyJ Clin Microbiol 2005 43:4357-62.10.1128/JCM.43.9.4357-4362.200516145077 [Google Scholar] [CrossRef] [PubMed]
[23]. Thangappah RB, Paramasivan CN, Narayanan S, Evaluating PCR, culture & histopathology in the diagnosis of female genital tuberculosisIndian J Med Res 2011 134:40-46. [Google Scholar]
[24]. Greco S, Girardi E, Navarra A, Saltini C, Current evidence on diagnostic accuracy of commercially based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosisThorax 2006 61(9):783-90.10.1136/thx.2005.05490816738037 [Google Scholar] [CrossRef] [PubMed]
[25]. Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, A systematic review of rapid diagnostic tests for the detection of tuberculosis infectionHealth Technol Assess 2007 11(3):1-196.10.3310/hta1103017266837 [Google Scholar] [CrossRef] [PubMed]
[26]. Guerra RL, Hooper NM, Baker JF, Alborz R, Armstrong DT, Maltas G, Use of the amplified mycobacterium tuberculosis direct test in a public health laboratory: test performance and impact on clinical careChest 2007 132:946-51.10.1378/chest.06-295917573496 [Google Scholar] [CrossRef] [PubMed]
[27]. Mirsaeidi M, Machado RF, Garcia JG, Schraufnagel DE, Nontuberculous mycobacterial disease mortality in the United States, 1999–2010: a population-based comparative studyPLoS One 2014 9:e9187910.1371/journal.pone.009187924632814 [Google Scholar] [CrossRef] [PubMed]
[28]. Adjemian J, Olivier KN, Seitz A, Holland S, Prevots R, Prevalence of pulmonary nontuberculous mycobacterial infections among U.S. Medicare beneficiaries, 1997-2007Am J Respir Crit Care Med 2012 185:881-86.10.1164/rccm.201111-2016OC22312016 [Google Scholar] [CrossRef] [PubMed]
[29]. Maurya AK, Nag VL, Kant S, Kushwaha RA, Kumar M, Singh AK, Prevalence of nontuberculous mycobacteria among extrapulmonary tuberculosis cases in tertiary care centers in Northern IndiaBioMed Research International 2015 2015:46540310.1155/2015/46540325883962 [Google Scholar] [CrossRef] [PubMed]
[30]. Myneedu VP, Verma AK, Bhalla M, Arora J, Reza S, Sah GC, Occurrence of non-tuberculous mycobacterium in clinical samples—a potential pathogenIndian Journal of Tuberculosis 2013 60(2):71-76. [Google Scholar]