Meningiomas are one of the most common primary tumours of the central nervous system accounting for 25 to 30% [1,2]. It arises from the meningothelial or arachnoidal cells and show characteristic attachment to the dura mater [3]. World Health Organization (WHO) classifies meningiomas in to 3 grades such as grade I, II, and III based on the histomorphology of the tumour [4]. Although, majority of meningiomas are classified as benign or low grade, it is very difficult to assess their behaviour as even low grade meningiomas tend to recur. The higher incidence of meningiomas among women [1] and the increased growth of tumour during pregnancy or following hormonal replacement therapy [1] has led various investigators to study the hormonal receptor status of meningiomas, it’s relation to the biological behaviour of the tumour and the scope for future therapeutic interventions of these tumours [3,4].
PR marker is classically associated with breast and endometrial carcinomas. Many studies have reported PR positivity in meningiomas [1,3,4]. Some studies have demonstrated that presence of PR is a favourable prognostic factor in meningiomas and have proven a positive association between the PR negativity and increased tumour recurrence rates [1]. Various authors have come up with different results based on their studies and hence the effect of hormones on the tumourigenesis or growth of meningiomas remains unclear [5]. This study was done to analyse the biological behaviour of meningiomas with respect to their PR status.
The aims and objectives of this study were to analyse the immunohistochemical incidence and distribution of PR expression in meningiomas, to correlate the percentage of expression of PR with respect to the clinicopathological parameters and to analyse the role of PR expression in prognosis of meningioma.
Materials and Methods
This was a retrospective and prospective study carried out for a period of three years (Jan 2013- Dec 2016) at a tertiary care hospital after obtaining clearance from the ethical committee. A total of 250 cases of meningiomas were reported during this period of which 157 and 93 cases were chosen retrospectively and prospectively respectively. Among these 250 cases of meningiomas paraffin blocks of 60 cases were chosen for immunohistochemical evaluation for PR. These cases were selected in such a way that it contained meningiomas occurring in both male and female patients, at various sites of occurrence and various age groups. This study included all the intracranial and intraspinal meningiomas of the three WHO grades and Recurrent meningiomas. All other tumours of meningeal origin such as haemangiopericytoma, haemangioblastoma and solitary fibrous tumours were not included in the study.
The PR expressions in these cases were compared with various clinical parameters such as age, sex, site of the tumour, rates of recurrence of tumour and with various pathological parameters such as WHO grades and variants of meningiomas. The sections were stained with monoclonal rabbit antihuman PR and poly excel Horse Raddish Peroxidase (HRP) with Diamino-Benzidene (DAB) detecting system purchased from pathnsitu belonging to lot number PR068. Sections from breast carcinoma stained for PR were taken as positive control. Negative controls samples were obtained by avoiding the staining with the primary antibody step during the staining procedure.
Interpretation and Scoring
The slides were assessed for the presence and cellular localisation of the PR immunohistochemical staining. PR characteristically shows nuclear receptor positivity. Non specific staining of the connective tissue and the cytoplasm is considered as negative. The percentage of cells that took up the stain were analysed and scored as follows: <1% cells- score 0; 2-10% cells- score 1; 11-50% cells- score 2; and >50% cells- score 3. PR staining in >1% of cells is considered positive [6].
Statistical Analysis
It was carried out using SPSS software version 17. Various tests used in the study were the chi-square test for discrete variables and the t-test for continuous variables. A level of significance 95% confidence interval with a p-value of less than 0.05 were considered as significant association between various factors analysed in the study.
Results
Immunohistochemical study for PR was done for 60 cases of meningiomas. A total of 40 cases (66.7%) of meningioma were positive for PR. The number of male and female patients selected for PR immunohistochemical study were 27 and 33 cases respectively of which 16/27 male cases (26.7%) and 24/33 female cases (40%) were found to be positive for PR. There was no difference in the degree of PR positivity in meningiomas among male and female patients. Hence, their association was not statistically significant with a p-value of 0.271.
The level of PR expression at various age groups of presentation of meningiomas were compared and given in [Table/Fig-1]. There was no difference in the degree of PR positivity in meningiomas among different age groups of presentation. Hence, their association were not statistically significant (p-value = 0.738).
Of all the 60 cases, 38/58 intracranial meningiomas and 2/2 of the intraspinal meningiomas were positive for PR. The various site of occurrence of meningiomas were compared with the level of PR expression and is given in [Table/Fig-2]. There was no difference in the degree of positivity of meningiomas among different location of meningiomas (p-value of 0.542).
Comparison of progesterone receptor expression in meningiomas among the different age groups at presentation.
| Age (years) | PR Positive(number ofcases & %) | PR Negative(number ofcases & %) | Total(number ofcases & %) |
|---|
| 21-30 | 4(6.7) | 2 (3.3) | 6 (10) |
| 31-40 | 6 (10) | 3(5) | 9 (15) |
| 41-50 | 11 (18.3) | 9 (15) | 20 (33.3) |
| 51-60 | 13(21.7) | 3 (5) | 16 (26.7) |
| 61- 70 | 4(6.7) | 2 (3.3) | 6 (10) |
| 71 and above | 2 (3.3) | 1 (1.7) | 3 (5) |
| Total | 40 (66.7) | 20 (33.3) | 60 (100) |
Statistical Test used: chi-square test (p-value= 0.738)
Comparison of progesterone receptor expression among the various sites of occurrence of meningiomas.
| Site | PR PositiveN (%) | PR NegativeN (%) | TotalN (%) |
|---|
| Intracranial | Convexity Meningiomas | 21 (35) | 16 (26.7) | 37 (61.7) |
| CP angle | 0 (0) | 1 (1.7) | 1 (1.7) |
| Intra ventricular | 0 (0) | 1 (1.7) | 1 (1.7) |
| Multiple Meningiomas | 0 (0) | 1 (1.7) | 1 (1.7) |
| Olfactory groove | 6 (10) | 1 (1.7) | 7 (11.7) |
| Para/Supra sellar | 2 (3.3) | 0 (0) | 2 (3.3) |
| Posterior fossa | 1 (1.7) | 0 (0) | 1 (1.7) |
| Sphenoid wing | 7 (11.7) | 0 (0) | 7 (11.7) |
| Tentorial sol | 1 (1.7) | 0 (0) | 1 (1.7) |
| Intraspinal | Spinal sol | 2 (3.3) | 0 (0) | 2 (3.3) |
| Total | 40 (66.7) | 20 (33.3) | 60 (100) |
Statistical Test Used: chi-square test (p-value= 0.542)
The PR expression in different histopathological types of meningiomas is given in [Table/Fig-3]. Among all types of meningiomas, meningothelial variant (40% of total PR positive cases) showed the maximum expression of PR followed by the transitional type (20% of total PR positive cases). One case of multiple meningioma (transitional type) in the cerebral convexity was encountered and it showed negativity for progesterone receptors.
Comparison of progesterone receptor expression among different histopathological types of meningiomas.
| Grade | HPE Types | Total Number of Cases | PR Positive(number ofcases & %) | PR Negative(number ofcases & %) |
|---|
| Grade I | Meningothelial Meningioma | 26 (43.3%) | 24 (40%) | 2 (3.3%) |
| Transitional Meningioma | 16 (26.7%) | 12 (20%) | 4 (6.7%) |
| Fibrous Meningioma | 1 (1.7%) | 1 (1.7%) | 0 (0%) |
| Lympho plasmacytic Meningioma | 1 (1.7%) | 0 (0%) | 1 (1.7%) |
| Microcystic Meningioma | 4 (6.7%) | 1 (1.7%) | 3 (5%) |
| Angiomatous Meningioma | 1 (1.7%) | 0 (0%) | 1 (1.7%) |
| Psammomatous Meningioma | 1 (1.7%) | 1 (1.7%) | 0 (0%) |
| Total | 50 (83.3%) | 39 (65%) | 11 (18.3%) |
| Grade II | Atypical Meningioma | 6 (10%) | 1 (1.7%) | 5 (8.3%) |
| Chordoid Meningioma | 1 (1.7%) | 0 (0%) | 1 (1.7%) |
| Clear cell Meningioma | 1 (1.7%) | 0 (0%) | 1 (1.7%) |
| Total | 8(13.3%) | 1(1.7%) | 7 (11.7%) |
| Grade III | Anaplastic Meningioma | 1 (1.7%) | 0 (0%) | 1 (1.7%) |
| Papillary Meningioma | 1 (1.7%) | 0 (0%) | 1 (1.7%) |
| Total | 2(3.3%) | 0 (0%) | 2(3.3%) |
| Total | | 60(100%) | 40(66.7%) | 20 (33.3%) |
Statistical Test Used: chi-square test (p-value= 0.00)
Among the 60 cases, 50, 8 and 2 cases belonged to grade I, II and III respectively. Their histopathological features are depicted in [Table/Fig-4] and PR positivity status is given in [Table/Fig-5]. In comparison with grade I meningiomas the immunohistochemical expression of PR in high grade (II and III) meningiomas were found to be from weak to absent, thereby showing a statistically significant (p-value= 0.00) association between low rates of expression of PR and increased tumour aggressiveness.
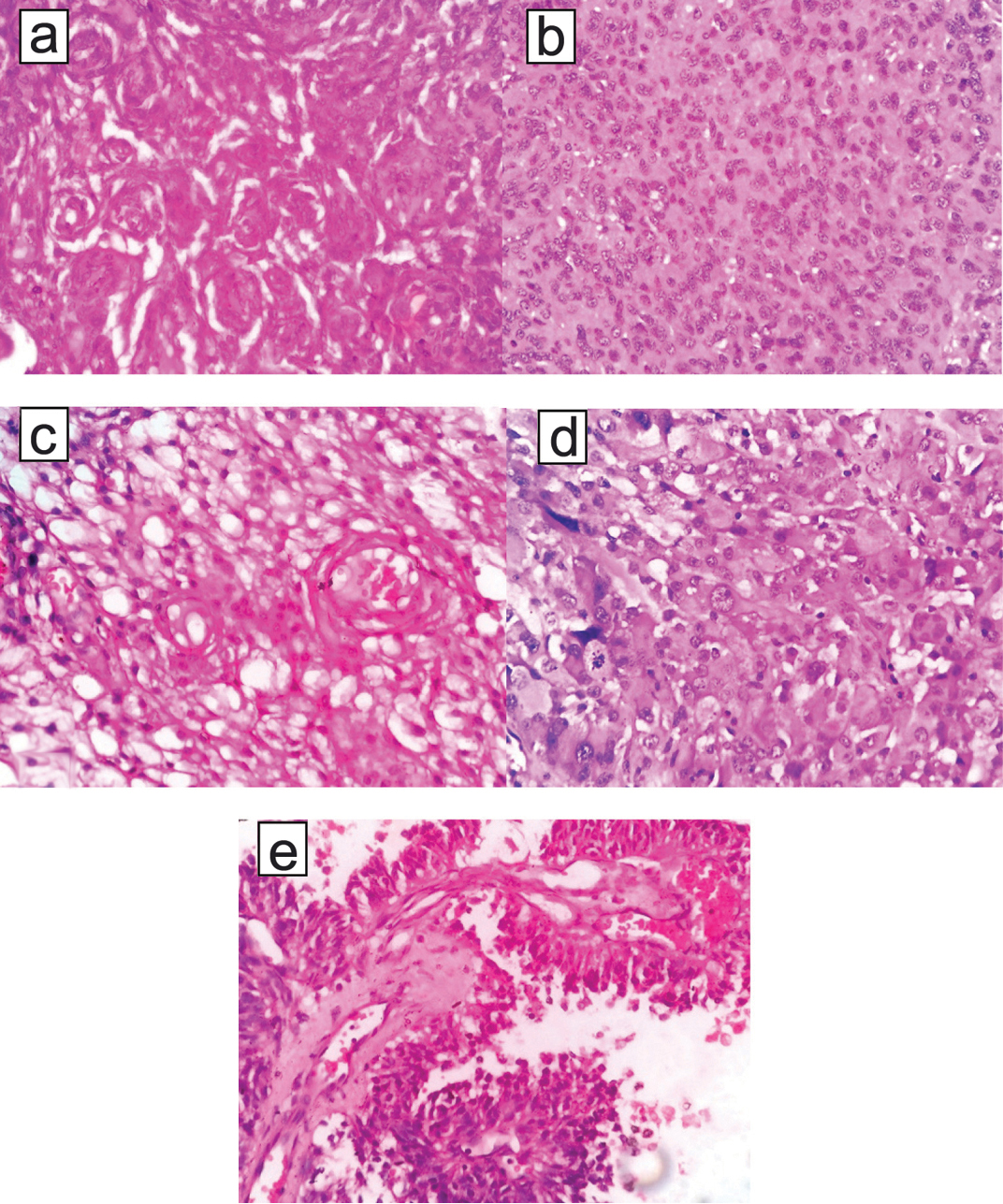
a) shows grade I meningothelial meningioma; (b,c) shows grade II meningiomas such as atypical meningioma & clear cell meningioma respectively; (d&e) shows anaplastic meningioma and papillary meninigoma respectively. a to e- (H&E; 400 X).
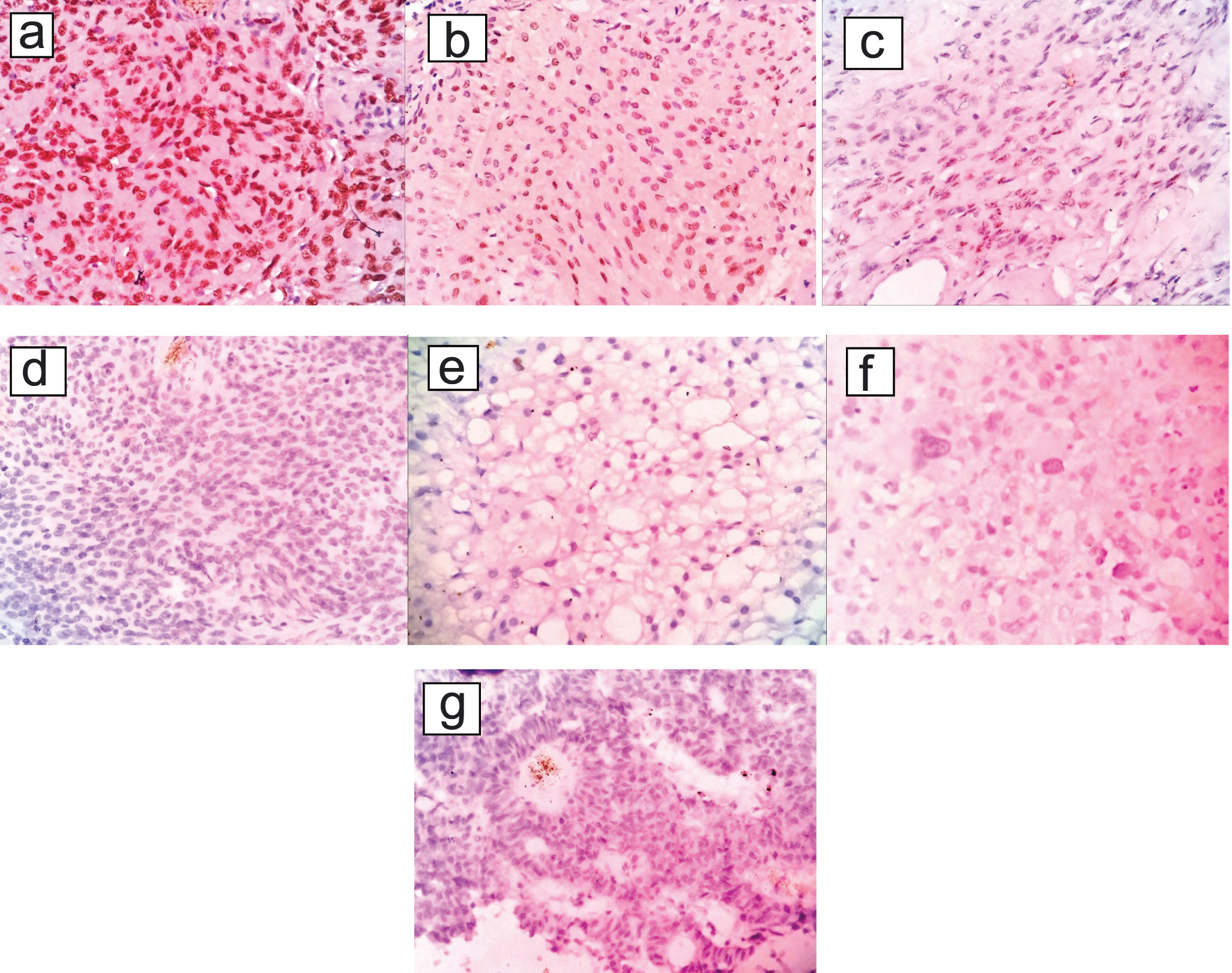
(a-c) shows grade I meningiomas exhibiting strong, moderate and weak intensity of positivity for progesterone receptors respectively; (d-g) shows grade II & III meningiomas such as atypical meningioma, clear cell meningioma, anaplastic meningioma and papillary meninigoma exhibiting negativity for progesterone receptors respectively. (a-f) (H&E; 400 X) and g) (H&E; 400 X).
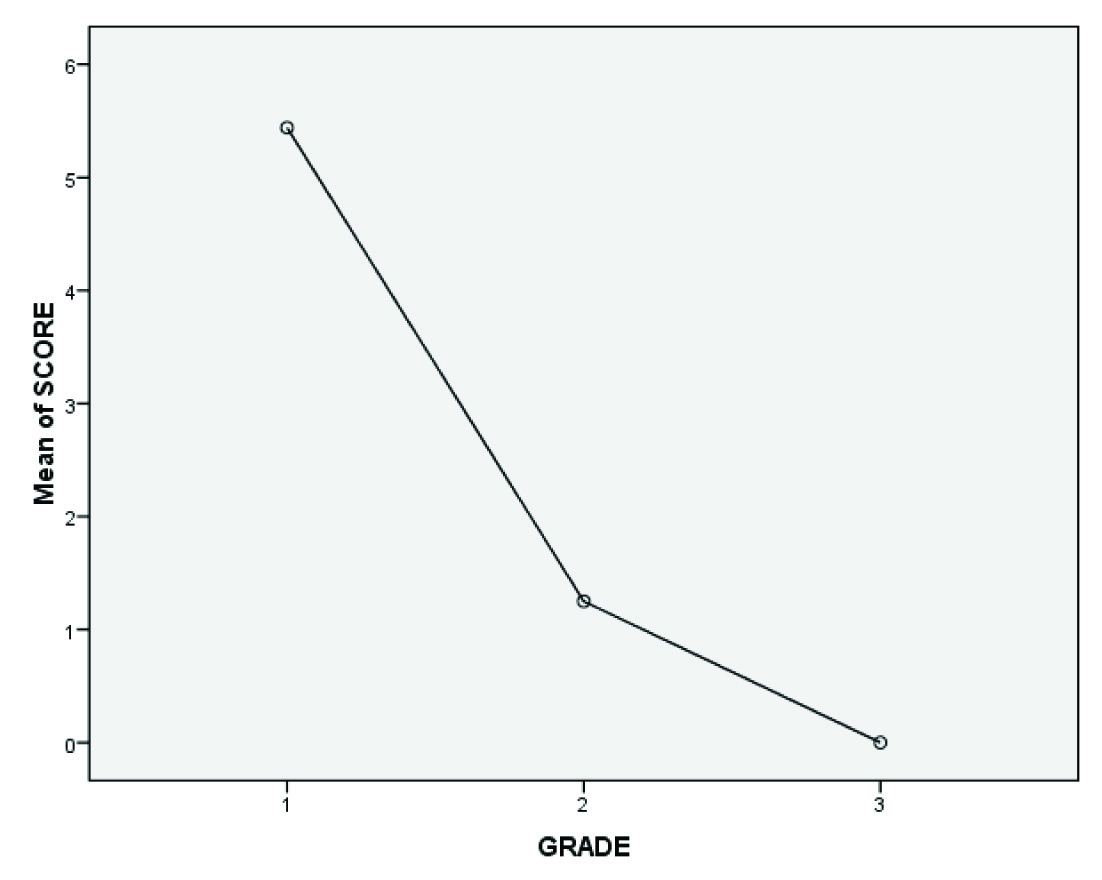
The mean cumulative PR positivity score (obtained by addition of the intensity score with the score for percentage of positive cells) also shows that the scores are high with grade I meningiomas (score of more than or equal to 2 to 8 with mean score for grade I being 5.5) and decreases as one moves through grade II to grade III (score of less than or equal to 1 with mean score for grade II = 1.2 and grade III = 0 – as most of the grade II and III meningiomas were weakly positive or negative for PR) as shown below in [Table/Fig-6].
Comparison of the mean cumulative score of progesterone receptor positivity among grade I, II and III meningiomas.
Mean score of each grade: grade I = 5.5, grade II = 1.2, grade III = 0
The recurrence rate among meningiomas in this study period of three years was 3.3% (7 cases). Of these seven cases, 2(28.6%), 4(57%) and 1(14%) cases belonged to grade I, II and III respectively. All these cases of recurrent meningiomas showed negativity for PR. The two cases of grade I meningiomas that showed recurrence had evidence of brain infiltration. Thereby, showing that loss of PR expression is associated with increased tumour aggressiveness and recurrence proving their association to be statistically significant p-value<0.001.
Discussion
Meningiomas are the second most common tumours of the central nervous system. The prevalence of histopathologically confirmed meningiomas is approximately found to be 97.5/100,000 [7]. There is a higher incidence of these tumours among female patients with the female:male ratio being 1.7:1 [2] to 3.5:1 [8]. Spinal meningiomas shows even a higher predilection for females as much as 90% [1]. Benign meningiomas are the most common in incidence accounting for more than 80% of cases. The atypical meningiomas accounts for 4.7% to 7.2% to as high as 20% of all meningiomas [9] and as quoted by Willis J et al., malignant/anaplastic meningiomas comprises between 1.0% and 2.8% [10]. More than 80% of meningiomas are supratentorial in location [11]. Less common sites include those arising from the choroid plexus and CNS parenchyma itself [12,13]. One case of intraventricular meningioma was included in this study which forms one of the less common sites of occurrence of meningiomas.
Radiation exposure and hormonal influence forms the two most important risk factors for the development of meningiomas [14]. For example, females show an increased incidence of meningiomas and it occurs rarely before puberty or after menopause, corresponding to the reproductive years with time of maximal hormonal activity [15-17]. The above findings correlated well with this particular study also. Some women suffers increase of symptoms during the luteal phase of menstrual cycle due to presence of functional progesterone rather than oestrogen receptors in majority of meningiomas [16,18]. It is said by Korhonen K et al., that 40%, 88% and 39% of meningiomas have Oestrogen Receptors (ER), Progesterone Receptors (PR), and Androgen Receptors (AR) respectively [19].
PR are members of steroid receptor family with a nuclear localisation identified by immunohistochemical staining and their expression in meningioma is said to be a favourable prognostic factor in various studies [3,20]. This study also came across similar findings. Hilbig A et al., Piquer J et al., and Fewings P et al., demonstrated that tumours with increased necrosis and proliferation rate had lower levels of progesterone expression [21-23].
Some studies have given contradictory results. For example while Perry A et al., and Jay JR et al., showed the effect of hormonal influence on growth of the tumour, Adams EF et al., suggested that there was no role of PR in the growth of meningiomas [5,24,25]. Though, majority of meningiomas express PR it’s been a question of debate whether these receptors are functional. Many studies involving anti-progesterone drug mifepristone and cell cultures of meningiomas with the progesterone have demonstrated that these receptors are functional. However, the results of invitro studies are variable [5,25].
The ER expression in meningiomas were very scarce and those few meningiomas that exhibit ER positivity have not been positive for PR and they tend to be of higher grade meningiomas with aggressive behaviour [26,27]. Taghipour M et al., (51 cases) and Hilbig A et al., (116 cases) in their study states that none of their cases tested for ER expression came out to be positive but majority of the cases were found to express PR [4,21]. Due to the very lower rate or absence of expression of oestrogen receptors in meningiomas, they were not done in this study. The rate of expression of Ki67 (MIB-1antiboby labeling) has been associated with prognosis of meningiomas. Greater the Ki67, lesser the PR and more likely those tumours will recur [1,3,28].
The magnitude of PR expression among various studies ranges from 50-70%, our study also correlates well with these findings. The PR expression in this current study (66.7%) correlated well with other studies done by Roser F et al., (53.5%), Taghipour M et al., (68.6%) and Hilbig A et al., (53%) [1,4,21].
Taghipour M et al., and Hilbig A et al., had said in their study that there is no association between the magnitude of PR expression levels and age or site of the meningiomas [4,21]. This study also showed similar results. The finding that there were a slightly higher proportion of PR positivity among females when compared to males goes well with other studies also [3,4].
In the study done by Taghipour M et al., all the five cases of intraspinal meningiomas included in their study showed strong positivity for PR [4]. In our study also 2/2 cases (100% of cases in this study) of intraspinal meningiomas of grade I type showed strong PR expression. Due to the less number of these cases the results could not be generalised that intraspinal meningiomas have expression of PR and they were found to be statistically insignificant. PR expression in males and females among various grades in the current study are correlated with other studies done by Roser F et al., and Shayanfar N et al., as given in [Table/Fig-7] [1,3].
Comparison of PR immunohistochemical testing between current study and a similar study done by Roser F et al., & Shayanfar N et al., [1,3].
| Present Study | Shayanfar N et al., | Roser F et al., |
|---|
| Total Number Of Cases Tested For PR IHC | 60 (100 %) | 78(100 %) | 588(100 %) |
| PR Positivity In Males | 59.25% | 68% | 56% |
| PR Positivity In Females | 72.7% | 89% | 56% |
| PR Positivity In Grade IMaleFemale | 16/23 (69.5 %)23/27 (85 %) | - | 96/158 (60.7%)212/ 375 (56.5%) |
| PR Positivity In Grade IIMaleFemale | 0/3 (0%)1/5 (20%) | - | 13/28 (46.4 %)8/18 (44.4 %) |
| PR Positivity In Grade IIIMaleFemale | 0/1 (0%)0/1 (0%) | - | 0/6 (0%)1/3 (33.3%) |
According to Roser F et al., Shayanfar N et al., and Taghipour M et al., meningothelial meningiomas had higher expression of PR among the various types of meningiomas [1,3,4]. This study also showed similar results. Omulecka A et al., said that among all types of grade I meningiomas in their study meningothelial meningiomas had increased expression and fibrous type of meningiomas had the weakest expression of PR. This might be because the line of differentiation of cells in fibrous meningiomas are more towards a mesenchyme rather than epithelial like cells [29].
The cumulative score of PR expression was found to be higher in case of grade I meningiomas and the score decrease as one proceeds through grade II and III meningiomas. This finding of the current study correlated well with study done by Kandemir NO et al., [6]. Roser F et al., Shayanfar N et al., Taghipour M et al., and Hilbig A et al., said that in comparison with grade I meningiomas, the immunohistochemical expression of PR in grade II and III meningiomas were found to be from weak to absent [1,3,4,21]. There has been association between low rates of expression of PR and increased tumour aggressiveness. This finding correlated well with this study also.
Even benign meningiomas are very challenging to treat. The various treatment modalities include presurgical angiographic embolisation of tumour, followed by surgery and post surgical irradiation [30]. For some unknown reasons meningiomas were found to recur inspite of best therapeutic efforts.
The recurrence rates for benign (grade I), atypical (grade II) and malignant (grade III) meningiomas ranged from 7-25%, 29-52%, and 50-94% respectively in various studies such as Yang SY et al., Pasquier D et al., Palma L et al., & Perry A et al., [31-34]. The factors associated with increased recurrence in meningiomas includes location of tumour, extent of surgical resection of tumour, tumour histology and grading according to WHO grading system, proliferation associated markers such as Ki67, PR receptor status negativity [35].
Due to the factors such as ageing, presence of other medical problems or too large tumours and tumours located at unfavourable sites a successful surgical removal is not possible. Since majority of the meningiomas have been found to express PR, various in vitro studies [36-38] have shown that proliferation of meningiomas were inhibited by RU486 (mifepristone), a PR antagonist suggesting it as a treatment modality. Previously anti-progesterone therapy which was used to treat breast cancer patients has now been found to be useful in treating meningiomas [36,37].
In one study done by Grunberg SM et al., out of the 13 patients who were given mifepristone therapy, five patients showed reduction in tumour, three showed improvement without reduction in tumour and three showed enlargement of tumour regardless of the therapy. Two of the three cases that failed to respond were malignant meningiomas [36].
In the other study done by Lamberts SWJ et al., with a study group of 10 patients of inoperable cases of meningiomas four cases failed to respond to mifepristone therapy. PR study was not done in any of the above cases. May be the absence of PR led to the failed response to the treatment with mifepristone in some cases [37]. Preoperative administration of medroxy progesterone in patients with positive PR had a better clinical outcome compared to those without PR expression as showed by Walter LM et al., [38]. However, only marginal responses were obtained from various small clinical trials [39]. Some of the prospective studies are still under trail awaiting completion. Hence, a more detailed research work is needed to study these receptor expressions in meningiomas not only at the immunohistochemical level but also at the genetic level to know their functional status in order that they could be therapeutically targeted.
Limitation
The limitations of this particular study are that only the expression of PR was evaluated. Whether these receptors are functional and whether they respond to antimifepristone therapy was not evaluated.
Conclusion
After an extensive workup on the histomorphological series of meningiomas it is concluded that immunohistochemical analysis of meningiomas for PR helped to predict the behaviour of tumour. Most of the meningiomas that expressed PR have had increased cure rates with reduced chances of recurrence and those that did not express PR showed higher grades of tumour. Hence, the expression of PR even in small numbers of meningiomas tumour cells have been considered as a useful prognostic tool in correlation with other histopathological features such as histological grade of the tumour, presence or absence of brain invasion and mitotic rates in assessing the behaviour of the tumour. Targeted therapy with PR antagonists may be considered in the treatment of meningiomas and it requires a more detailed research and investigation.
Statistical Test used: chi-square test (p-value= 0.738)
Statistical Test Used: chi-square test (p-value= 0.542)
Statistical Test Used: chi-square test (p-value= 0.00)