Penicillin Resistant Pneumococcal Meningitis in Paediatric Trauma Patient
Vijeta Bajpai1, Aishwarya Govindaswamy2, Sushma Sagar3, Purva Mathur4
1 Senior Resident, Department of Microbiology, Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, India.
2 Senior Resident, Department of Microbiology, Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, India.
3 Professor, Department of Trauma and Surgery, Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, India.
4 Professor, Department of Microbiology, Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Purva Mathur, Professor, Department of Microbiology, Jai Prakash Narayan Apex Trauma Centre, All India Institute of Medical Sciences, New Delhi-110029, India.
E-mail: purvamathur@yahoo.co.in
Pneumococcal meningitis is a life-threatening infection, requiring prompt diagnosis and effective treatment. The approach to therapy in patients with pneumococcal meningitis has changed considerably over the past 20 years because of emerging penicillin resistant pneumococcal meningitis. We report a case of Penicillin resistant pneumococcal meningitis in a six-year-old child after skull trauma injury. The patient responded well to injection ceftriaxone and injection vancomycin therapy and recovered completely without sequelae.
Meningeal syndrome, Streptococcus pneumoniae, Subcondylar mandible fracture, Trauma surgery
Case Report
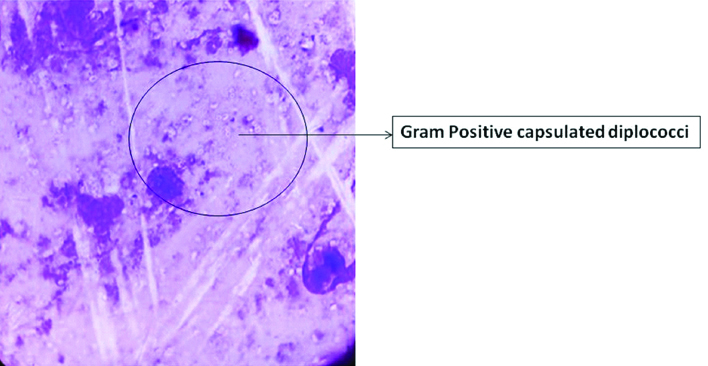
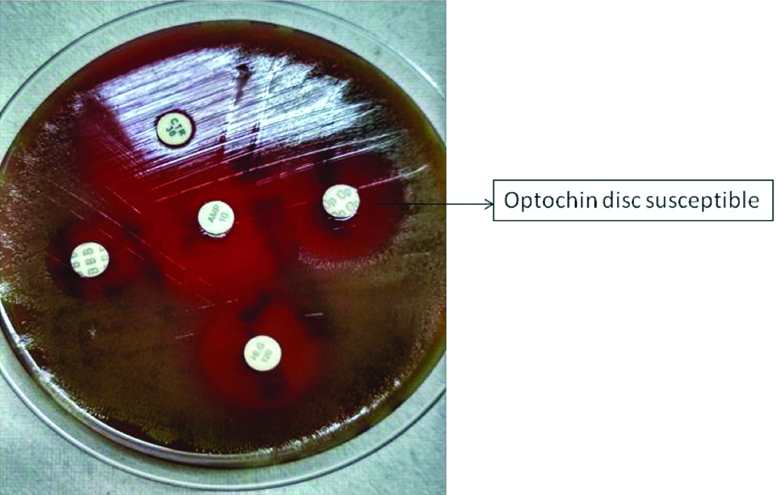
A six-year-old boy was admitted to the emergency unit of trauma centre after falling from a height. The child suffered from polytrauma including basal skull fracture, right para-symphysis and right subcondylar mandible fracture along with fracture of both bone of right forearm. At the time of admission, there was no history of fever, vomiting, seizure, neck rigidity, chest pain, abdominal pain. X-ray chest showed no signs of pneumothorax, haemothorax, cavity or consolidation. In view of multiple injuries injection Tetanus 0.5 mL (IM) stat and injection Augmentin 500 mg started and call given to surgeons for operative intervention of skull fracture and other maxillofacial injuries. Within 48 hours of admission, the patient developed high grade fever (104°F), asthenia, lethargy, nasal discharge and vomiting. Physical examination revealed clear meningeal syndrome with neck rigidity and positive Kernig and Brudzinski signs. In view of suspicion of meningitis, lumber puncture was done and Cerebrospinal Fluid (CSF) sample was sent for cell count, biochemical analysis, and microbiological culture. CSF was turbid in appearance and had pleocytosis (350 leucocytes/microliter, of which 75% were neutrophils). Biochemical analysis of CSF showed decreased glucose (35 mg/dL) and elevated protein (136 mg/dL) level. Microscopy of the gram stained smear of CSF showed Gram-positive lanceolate shaped cocci, mostly in pairs, capsulated along with numerous pus cells [Table/Fig-1]. The CSF sample was cultured on blood agar, chocolate agar and MacConkey’s agar and incubated at 37°C in a 5% CO2 atmosphere. After 24 hour of incubation, cultures of CSF yielded pure growth of catalase-negative, alpha haemolytic non mucoid small translucent colonies appeared on blood agar. The growth on chocolate agar was surrounded by a green zone [Table/Fig-2]. However, the organism failed to grow on MacConkey’s agar. The isolate was also presumptively identified as Streptococci Pneumoniae (S. pneumoniae) on the basis sensitivity of 5-μg optochin disk which showed a diameter inhibition zone of 18-mm after incubation in an atmosphere of increased CO2 [Table/Fig-3]. Further confirmatory identification and antimicrobial sensitivity were done by automated instrument VITEK -2 ST-03 card according to the manufacturer’s instructions. Based on biochemical tests in VITEK -2 (Bile solubility positive, optochin susceptibility present, and inulin fermentation), it was identified as S. pneumoniae. However, serotyping could not be done due to lack of antisera. Antimicrobial susceptibility of S. pneumoniae displayed Resistant (R) to penicillin G, Minimal Inhibitory Concentration (MIC=0.25 μg/mL); and erythromycin (MIC 4 μg/mL); Sensitive (S) to cefotaxime, (MIC ≤0.12 μg/mL), ceftriaxone (MIC 0.25 μg/mL); levofloxacin (MIC 0.5 μg/mL); moxifloxacin (MIC 0.12 μg/mL); clindamycin (MIC ≤0.25 μg/mL); linezoild (MIC ≤2 μg/mL); vancomycin (MIC=0.5 μg/mL); and rifampin (MIC=0.006 μg/mL). The patient was in antibiotic prophylaxis with injection augmentin; however, despite of this, the patient got sick. Therefore, the treatment was changed from augmentin to intravenous ceftriaxone and vancomycin (300 mg/kg/day). After 24-36 hour of therapy the patient improved symptomatically. A second CSF puncture performed after 48 hour was sterile. Two blood cultures were also sterile. Finally, the patient had an uneventful hospital stay and was discharged on day 13, after completely recovering. Follow-up at one week was favourable, with no neurological and auditory sequelae.
Gram stain picture of CSF sample of patient.
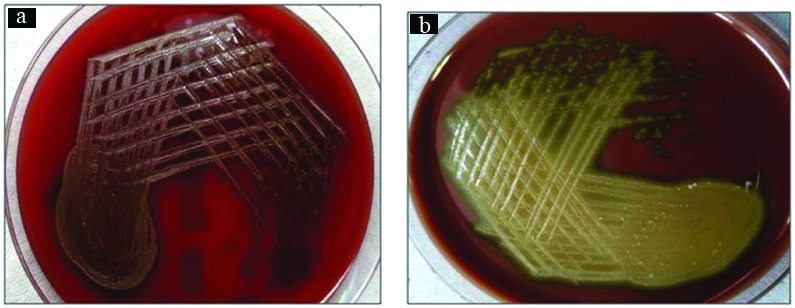
Streptococci pneumoniae growth in blood and chocolate agar.
a) Blood agar showing growth of, alpha hemolytic non mucoid small translucent colonies; b) Chocolate agar: Alpha hemolytic non mucoid small translucent colonies, surrounded by a green zone
Optochin disc sensitivity testing for Streptococcus pneumoniae.
Discussion
Traumatic brain injury is a frequent reason for admission to paediatric emergency services. In severe cases, with basilar skull fracture, bacterial meningitis is a serious and potentially fatal complication to be considered [1]. Bacterial meningitis is a complication to be considered in head injury with basilar skull fracture, particularly when associated with CSF leakage, and is usually a serious condition [2]. Pneumococcal meningitis is one of the most common cause of bacterial meningitis in children [3]. Meningeal involvement is due to seeding of S. pneumoniae bacteremia and usually the most common mode of acquiring of meningitis. Less frequently, a breach in dura associated with head injury with a skull fracture and neurosurgery can also lead to spread of S. pneumoniae from infected paranasal sinuses, mastoid, and middle ear [3,4].
Pneumococcal meningitis is defined as isolation of S. pneumoniae from CSF with increased CSF white cell count >10 cells per mm3 [5]. The risk of developing pneumococcal meningitis is highest among young children, older adults, persons with chronic illnesses, and immunocompromised individuals [6]. It accounts for 34.3% to 43.5% of invasive pneumococcal disease in India [3,4]. Even with advances in medical care, the case-fatality rate has a range of 16%-37% in adults and 1%-2.6% in children. Those who survived the disease have a 30%-52% risk of neurological sequelae [6]. Pneumococcal meningitis is a life-threatening infection, requiring prompt diagnosis and effective treatment. Penicillin was considered as standard drug of choice for treating pneumococcal infections for many years until increasing global resistance to penicillin. In the past, S. pneumoniae was uniformly susceptible to penicillin with a MIC ≤0.06 μg/mL. According to current CLSI guidelines, Penicillin susceptibility break points for S. pneumoniae is <0.06 μg/mL, 0.12–1 μg/mL and >2 μg/mL for susceptibility, intermediate susceptibility and resistance, respectively [7,8].
Resistance to penicillin had increased over time amongst both meningeal and non-meningeal pneumococcal infections indicating the need for new strategies for effective management. Prevalence of penicillin resistance in S. pneumoniae ranges from 20% to 30% in Brazil and Argentina [8]; approximately 40% in the USA; to over 50% in Asia and Africa, with maximum rates approaching or above 80% in Taiwan and China [9]. The occurrence of antibiotic resistance varies in different studies from various centers in India. In a study from a tertiary care center in Southern India, penicillin and cefotaxime resistant were present in 43.7% and 14.9% pneumococcal strains respectively [9]. Study had also shown increased penicillin resistance of pneumococcal meningeal isolates, from <10% in 2008 to over 40% by 2016 [9].
Emerging trend of penicillin-resistant pneumococcal strains showed that parenteral administration of penicillin did not attain appropriate CSF concentrations to eradicate strains that are resistant to penicillin. Therefore, penicillin is not recommended for empiric therapy of patients with suspected pneumococcal meningitis and recommended empiric therapy is the combination of vancomycin plus a third-generation cephalosporin (either cefotaxime or ceftriaxone).
The Indian Council of Medical Research’s recently released guidelines on antimicrobial use in common syndromes recommend the use of ceftriaxone with ampicillin in the treatment of acute bacterial meningitis due to the low level of penicillin resistance in pneumococcal infections [10]. Penicillin resistance in the present case report pneumococcal isolate leads us to believe that it would be prudent to add vancomycin to ceftriaxone for initial empirical treatment of acute bacterial meningitis till culture reports become available, after which antibiotics can be administered based on results of susceptibility testing. Pneumococcal meningitis carries a significant morbidity and mortality despite the availability of effective antimicrobial therapy. Based on current knowledge of in vitro susceptibility patterns, the approach to therapy of pneumococcal meningitis must include consideration that antimicrobial resistance is likely.
Conclusion
The present case report emphasises the need to use combination therapy with vancomycin added to cefotaxime or ceftriaxone for all surgical patients with pneumococcal meningitis until the treatment regimen can be modified using results of susceptibility testing.
[1]. Santos SF, Rodrigues F, Dias A, Costa JA, Correia A, Oliveira G, Post-traumatic meningitis in children: eleven years’ analysisActa Med Port 2011 24(3):391-98. [Google Scholar]
[2]. Wei BPC, Shepherd RK, Robins-Browne RM, Clark GM, O’Leary SJ, Effects of inner ear trauma on the risk of pneumococcal meningitisArch Otolaryngol Neck Surg 2007 133(3):250-59.10.1001/archotol.133.3.25017372082 [Google Scholar] [CrossRef] [PubMed]
[3]. Thomas K, Mukkai Kesavan L, Veeraraghavan B, Jasmine S, Jude J, Shubankar M, Invasive pneumococcal disease associated with high case fatality in IndiaJ Clin Epidemiol 2013 66(1):36-43.10.1016/j.jclinepi.2012.04.00623177893 [Google Scholar] [CrossRef] [PubMed]
[4]. Shah AS, Nisarga R, Ravi Kumar KL, Hubler R, Herrera G, Kilgore PE, Establishment of population-based surveillance for invasive pneumococcal disease in Bangalore, IndiaIndian J Med Sci 2009 63(11):498-507.10.4103/0019-5359.5887920075551 [Google Scholar] [CrossRef] [PubMed]
[5]. Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J, Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case seriesLancet Neurol 2006 5(2):123-29.10.1016/S1474-4422(05)70288-X [Google Scholar] [CrossRef]
[6]. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States [Internet]. [cited 2018 Feb 23]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4822508/ [Google Scholar]
[7]. Mantese OC, Paula A de, Almeida VVP, Aguiar PADF de, Wolkers PCB, Alvares JR, Prevalence of serotypes and antimicrobial resistance of invasive strains of pneumococcus in children: analysis of 9 yearsJ Paediatr (Rio J) 2009 85(6):495-502.10.2223/JPED.195019859623 [Google Scholar] [CrossRef] [PubMed]
[8]. m100ed28_sample.pdf [Internet]. [cited 2018 Apr 2]. Available from: https://clsi.org/media/1930/m100ed28_sample.pdf [Google Scholar]
[9]. Verghese VP, Veeraraghavan B, Jayaraman R, Varghese R, Neeravi A, Jayaraman Y, Increasing incidence of penicillin- and cefotaxime-resistant Streptococcus pneumoniae causing meningitis in India: Time for revision of treatment guidelines?Indian J Med Microbiol 2017 35(2):228 [Google Scholar]
[10]. Indian Council of Medical Research. Department of Health Research, New Delhi, India, 2017. Treatment Guidelines for Antimicrobial Use in Common Syndromes. Available from: http://www.icmr.nic.in/guidelines/treatment%20guidelines%20for%20antimicrobial.pdf. [Last accessed on 2017 Mar 13] [Google Scholar]