Hydatid disease caused by Echinococcus granulosus is a common occurrence in liver and lungs. Isolated renal hydatid cyst without liver or lung involvement is extremely rare. We present a case of solitary renal hydatid disease diagnosed radiologically as a renal malignancy and intraoperatively suspected as a case of renal tuberculosis due to the extensive necrotic material and as the patient was a treated case of pulmonary tuberculosis. Diagnosis of renal hydatid disease was confirmed on histopathology.
Case Report
A 75-year-old female presented with pain abdomen since two months. She had past history of pulmonary tuberculosis for which she had taken treatment for six months. Clinically, renal tuberculosis and malignancy were considered as differential diagnosis. Ultrasonography of abdomen and pelvis showed a cystic renal mass suggestive of malignancy. The patient was operated and intraoperatively suspected to have renal tuberculosis. Nephrectomy specimen was sent for histopathological examination.
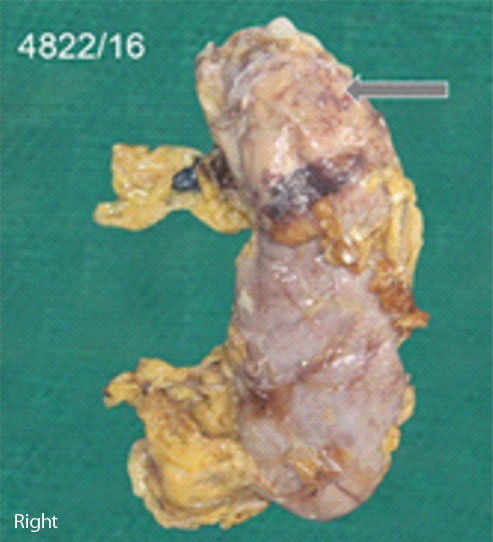
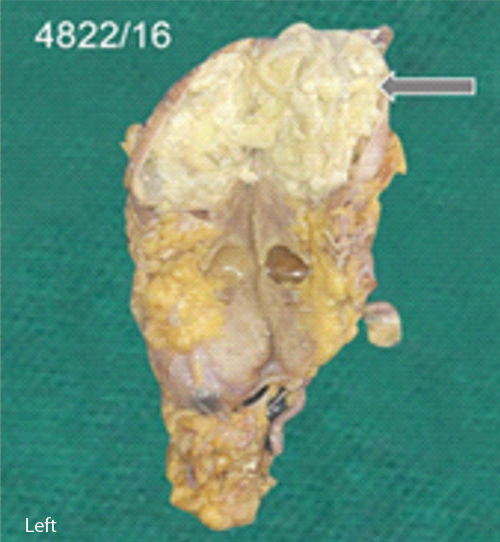
Nephrectomy specimen was measuring 15×10×3 cm with a solitary cystic lesion at the upper pole [Table/Fig-1a]. Cut section of the kidney [Table/Fig-1b] showed a distorted architecture. Cystic cavity filled with necrotic material was noted with a thickened cyst wall. Multiple sections were submitted from the cyst wall and necrotic material. Cyst contents were sent for cytological examination.
External surface of kidney.
Cut section, dilated pelvicalyceal system at one pole and filled with white cheesy material.
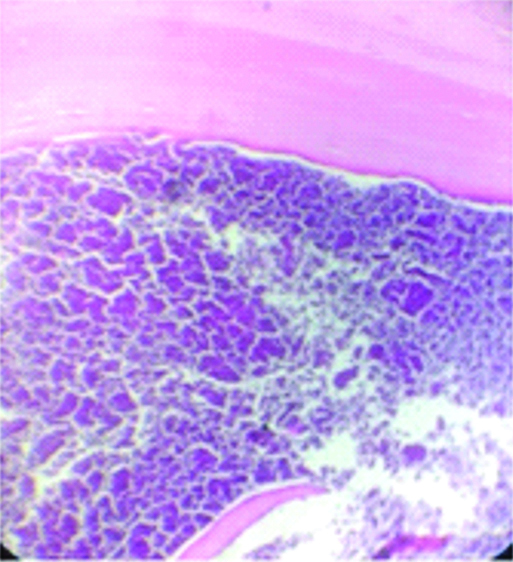
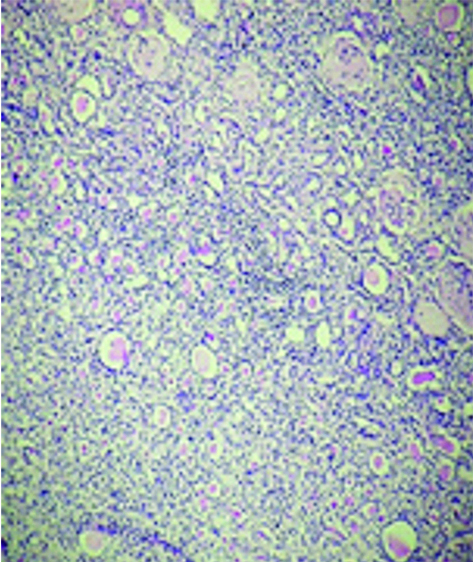
Haematoxylin and Eosin stained sections showed a lamellated eosinophilic layered membranous structure suggestive of cuticle and extensive areas of calcification [Table/Fig-2a]. Also, noted structure of kidney comprised of glomeruli, tubules, interstitium and blood vessels with thyroidisation of tubules and scant lymphocytic infiltration [Table/Fig-2b]. There was no evidence of granulomas or multinucleated giant cells or inflammatory cell infiltration. Ziehl Neelsen stain for acid fast bacilli was negative.
Cyst wall showing lamellated acellular eosinophilic cuticle and amorphous necrotic debris with calcified material noted beneath the cuticle; (H&E, x400).
Structure of kidney comprised of glomeruli, tubules, interstitium and blood vessels with thyroidisation of tubules and scant lymphocytic infiltration; (H&E, x100).
The pale white cheesy necrotic material was scraped from the cyst wall of formalin fixed nephrectomy specimen and smears were prepared. These smears showed no evidence of hooklets. Then, 20 mL of cyst content was centrifuged at 1500 rpm for 15 minutes and sediment has been subjected for cytospin preparation (200 mL) after decanting superficial fluid and cytospin smears (30) were stained using H&E staining which showed the hooklets and protoscolices [Table/Fig-3].
Arrows indicating hooklets of Echinococcus granulosus (H&E, 400x).

Discussion
Hydatid disease is caused by Echinococcus granulosus larval form [1]. It is endemic in Africa, Latin America, Southeast Asia where the rearing of sheep and cattle is common [2,3]. Dog is the definitive host, sheep is the intermediate host and humans are accidental intermediate hosts [1-4]. The liver is the most commonly involved site because of its defensive role followed by lungs [3,5,6]. Liver and lungs together represent 80-90% and the rest is constituted by other organs where in the kidney constitutes less than 2%, among which isolated renal hydatid disease is further rare [2-8]. The organism reaches the kidney, most commonly involved among the genitourinary tract, through blood stream, lymph glands or by direct invasion [1].
As patients are asymptomatic, most of the cases are detected late [9]. Hydatid disease can mimic malignancy both clinically and even on imaging studies.
Goeze, discovered the relationship between scolices of Echinococcus and formation of the cyst in the year 1782 [10]. The adult organism in the jejunum of dogs, measuring about 5 mm, excretes eggs in the faeces. Once these are accidentally ingested by humans, the embryos enter the portal circulation after penetrating the intestinal mucosa [1,2]. Females are most commonly affected and peak incidence is in the third decade to fifth decade [1,2].
Embryos may reside in any organ after penetrating the general circulation [9]. It appear in organs like liver (65%), lungs (25%), bones (5%), spleen (2%), heart (1%), pancreas (1%), and central nervous system (1%). Renal hydatid disease has an incidence of <2% of the total hydatid disease affecting humans which are due to the secondary invasion. Isolated renal hydatid disease is an extremely rare event [1,7,11]. The right lobe of the liver is affected commonly, due to the anatomical structure of liver vein. By similar logic, left kidney is involved preferentially as the left renal vein is shorter [1].
For many years hydatid cysts remain asymptomatic and grow enormously until detected [2,6]. At the time of presentation, it will be multiple daughter cysts in a single large cyst [3]. The wall of the cyst is comprised of an inner germinal layer and an outer laminated cuticle [2].
Renal hydatid cysts are not noticed unless they present with flank pain, haematuria, pyuria or intermittent fever and hydatiduria [1,4,5]. Even though being a pathognomonic sign, hydatiduria clinches the diagnosis in few cases ranging from 5-25% [1]. It involves passage of typical grape like material in the urine.
Eosinophilia, as found by many authors, is seen in about 20-50% cases and a further reduction in efficacy of eosinophilia as a diagnostic test is due to the false positive results in cases of concurrent parasitic infestations [1].
Radiologic studies are suggestive, but inconclusive, as they can mimic renal malignancies or an ureteropelvic junction obstruction. Ultrasound examination plays an important role in identifying the floating membranes, daughter cysts and the hydatid sand [1].
Serological tests are variable based on the location of the cyst and its maturity. Among the organs involved, lungs, spleen or kidney are associated with low levels of serum antibodies [3]. There is no serum antibody test with 100% sensitivity or specificity to detect the infection [2]. Degenerating cysts and cross reactions with parasitic infections like schistosomiasis leads to false negative and false positive serology results respectively [2]. Even in serologically positive cases, further evaluation with Intravenous Pyelography (IVP) and ultrasonography helps in making a diagnosis. Multimodality approach including clinical history, haematological, serological, ultrasonography, IVP and with a histopathological confirmation helps in optimally managing the patient and providing the best care for this curable disease [1,3].
In the present case, clinical diagnosis was considered as renal malignancy due to vague flank pain, older age group and ultrasonography examination findings. Blood and urine examination findings were insignificant. Based on the intraoperative findings and the fact that patient was a treated case of pulmonary tuberculosis a revised diagnosis of renal tuberculosis was made.
Grossly, cut section of the kidney had an appearance in line with caseous necrosis as seen in tuberculosis. But on microscopy, lamellated eosinophilic cuticle and extensive areas of calcification noted. Even after submitting tissue bits from multiple sites of the cyst wall, protoscolices or hooklets could not be demonstrated. So cytocentrifugation technique was utilized to demonstrate hooklets and protoscolices. A serological test was not done, as hydatid disease was not suspected clinically.
The radiological diagnosis was also not pointing towards the hydatid cyst as the probable cause and it was not even considered in the differential diagnosis given.
Conclusion
In view of this experience, it is recommended that a high degree of suspicion is always required for the consideration of isolated renal hydatid cyst disease among the differential diagnosis of renal cyst, as this disease has a definitive and curative treatment.
[1]. Chaudhary T, Biswas M, Mittal A, Sarpal R, Agrawal S, Shirazi N, Isolated cystic renal echinococcosis: a rare case reportInt Surg J 2016 3(3):1627-29.10.18203/2349-2902.isj20162760 [Google Scholar] [CrossRef]
[2]. Choi H, Park JY, Kim J, Moon DG, Lee J, Bae JH, Primary renal hydatid cyst: mis-interpretation as a renal malignancyKorean J Parasitol 2014 52(3):295-98.10.3347/kjp.2014.52.3.29525031471 [Google Scholar] [CrossRef] [PubMed]
[3]. Göğüş C, Safak M, Baltaci S, Türkölmez K, Isolated renal hydatidosis: experience with 20 casesJ Urol [Internet] 2003 169(1):186-89.10.1016/S0022-5347(05)64064-5 [Google Scholar] [CrossRef]
[4]. Horchani A, Nouira Y, Kbaier I, Attyaoui F, Zribi AS, Hydatid cyst of the kidney. a report of 147 controlled casesEuropean Urology 2000 38:461-67.10.1159/00002032511025387 [Google Scholar] [CrossRef] [PubMed]
[5]. Rexiati M, Mutalifu A, Azhati B, Wang W, Yang H, Sheyhedin I, Diagnosis and surgical treatment of renal hydatid disease: a retrospective analysis of 30 casesPlos One 2014 9(5):1-7.10.1371/journal.pone.009660224796329 [Google Scholar] [CrossRef] [PubMed]
[6]. Vats P, Jain A, Sachan S, Chaurasia R, Gupta V, Primary hydatid cyst of kidney: a rare entityJ Evolution Med Dent Sci 2016 5(14):674-76.10.14260/jemds/2016/154 [Google Scholar] [CrossRef]
[7]. Gossios K, Kontoyiannis D, Dascalogiannaki M, Gourtsoyiannis N, Uncommon locations of hydatid disease: CT appearancesEuropean Radiology 1997 7(8):1303-08.10.1007/s0033000502939377519 [Google Scholar] [CrossRef] [PubMed]
[8]. Goel M, Agarwal M, Misra A, Percutaneous drainage of renal hydatid cyst: early Results and follow-upBritish Journal of Urology 1995 75(6):724-28.10.1111/j.1464-410X.1995.tb07379.x7613827 [Google Scholar] [CrossRef] [PubMed]
[9]. Zmerli S, Ayed M, Horchani A, Chami I, El Ouakdi M, Ben Slama M, Hydatid cyst of the kidney: diagnosis and treatmentWorld Journal of Surgery 2001 25(1):68-74.10.1007/s00268002000911213158 [Google Scholar] [CrossRef] [PubMed]
[10]. Papadimitriou J, Mandrekas A, The surgical treatment of hydatid disease of the liverBritish Journal of Surgery 1970 57(6):431-33.10.1002/bjs.18005706075431573 [Google Scholar] [CrossRef] [PubMed]
[11]. Sachar S, Goyal S, Goyal S, Sangwan S, Uncommon locations and presentations of hydatid cystAnn Med Health Sci Res 2014 4(3):447-52.10.4103/2141-9248.13347624971224 [Google Scholar] [CrossRef] [PubMed]