There has been an increase in incidence of infections caused by fungal aetiology. Mycotic infections may aggravate or add to the underlying disease process or by themselves be responsible for major distress, disability, morbidity or life-threating situations. For many years only a few genera of fungi were known to cause human infections. However, an increase in size of population at risk, along with the ubiquitous presence of fungi has lead to a great expansion in the fungal aetiology [1]. Genera and species once known to be commensals are increasingly implicated in disease. The emergence of these opportunistic infections has created a challenge in diagnosis and management of fungal infections and emphasised the importance of medical mycology. The protocols for culture and identification of which require dedicated effort and high index of suspicion on the part of the clinical microbiologist.
Mycotic agents of disease could morphologically be moulds or yeasts. Amongst the yeasts, the genera could be true yeasts or yeast-like and some genera resembling yeasts. Yeast-like genera are as designated as they are not true yeasts and morphologically show yeast and pseudo-hyphal forms. Of these yeast-like genera, the genus Candida is the most common opportunistic pathogen, research on which is quite comprehensive and ongoing. The other yeast and yeast-like genera include Aureobasidium, Geotrichum, Malassezia, Pichia, Prototheca, Sporothrix and Tricosporon to name a few [2,3]. Among these Trichosporon spp. could be misreported as non-albicans Candida [4]. The isolation and identification of these NCYG is not much reported. They need more tests and identification is tedious; however, the clinical significance of these is ever-increasing and they are the emerging opportunistic pathogens. This study was planned with the aim to find the prevalence of these NCYG in clinical samples; To isolate and identify and speciate these genera.
Materials and Methods
This was a cross-sectional study carried out in the department of Microbiology of a tertiary care hospital in western Maharashtra. Samples received for mycology cultures from various clinical samples over a period of one year i.e., From January 2014 to December 2014; were included in the study.
Clinical suspected infections were classified as Mucocutaneous Infections (MCC), Urinary Tract Infections (UTI), Deep Seated Infections (DSI) and Blood Stream Infections (BSI). Samples for Mucocutaneous infections included skin, nail, vaginal secretions, ear discharge and pus from wound. Urine samples for UTI. DSI mainly included sputum, pleural fluid and broncho-alveolar lavages. BSI samples included blood and Central Venous Pressure (CVP) tips.
Samples were processed as per standard protocol for fungal follow-up. This included Microscopy, Culture, phenotypic manual identification tests and automated identification system VITEK2 Compact (Automated ID/AST by bioMerieux). Microscopy included wet mount, KOH mount, negative staining and Gram staining. All samples were cultured on four slants of Sabouraud Dextrose Agar (SDA); two with antibiotics and two without antibiotics. In addition, for mucocutaneous sample cultures were set up on SDA with olive oil at room temperatures for lipophilic fungi. One of each slant was incubated at 37°C and the other set at room temperature. Weekly readings were taken for four weeks. Positive cultures were identified by wet mount, gram stain, germ-tube test, CHROM agar urease test and VITEK 2 Compact system using YSTID cards [2,3].
Results
A total of 1982 samples from various clinical departments were included in the study. Amongst these fungal growths was obtained in 603 (30.42%) samples while the rest 1379 (69.58%) were negative for fungal growth [Table/Fig-1].
Details of samples and culture results.
| Total samples included | 1982 |
| Culture positive for fungal pathogens | 603 (30.42%) |
| Culture negative for fungal pathogens | 1379 (69.58%) |
Among 603, 224 (37.15%) were identified as moulds. Yeast and yeast-like were identified in 379 (62.85%) isolates [Table/Fig-2].
Details of isolates among Culture positive samples.
| Culture positive samples | Moulds/filamentous isolates | Yeasts and yeast-like isolates |
|---|
| 603 | 224 (37.15%) | 379 (62.85%) |
Yeast and yeast-like isolates: Among the 379 isolates, 324 (85.47%) were identified as Candida isolates, three as true yeasts (0.79% all Cryptococcus sp.). The remaining 52 (13.74%) were identified as NCYG i.e., yeast and yeast-like genera other than Candida [Table/Fig-3]. Further, among Candida species, 139 (42.90%) were C.albicans and 185 (57.10%) were non-albicans Candida. The non-albicans Candida was speciated as C.tropicalis-98, C.parapsilosis-49, C.glabrata-17, C.krusei-9, C.haemolounii-7, C.lusitanae-3, and C.famata-2 [Table/Fig-4].
Details of yeast and yeast-like isolates.
| Total yeast and yeast-like isolates | 379 |
| True yeasts | 03 (0.79%) |
| Candida isolates | 324 (85.47%) |
| NCYG | 52 (13.74%) |
Species distribution of Candida isolates.
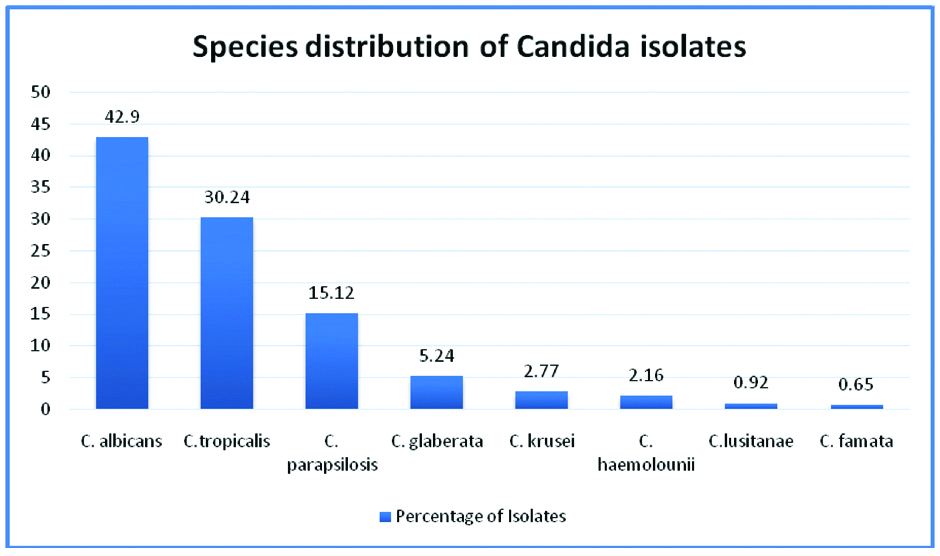
Thus 8.62% (52/603) of total culture positive were NCYG. Among yeast-like isolates the isolation rate was 13.72% (52/379). Among these isolates, 24 (46.15%) belonged to the genus Trichosporon. The next was Malassezia 14 (26.92%). The remaining were Sporothrix three, Pichia norvigensis three, two each Rhodotorula and Stephanoascusciferii, one of Protothecazopfii and others three.
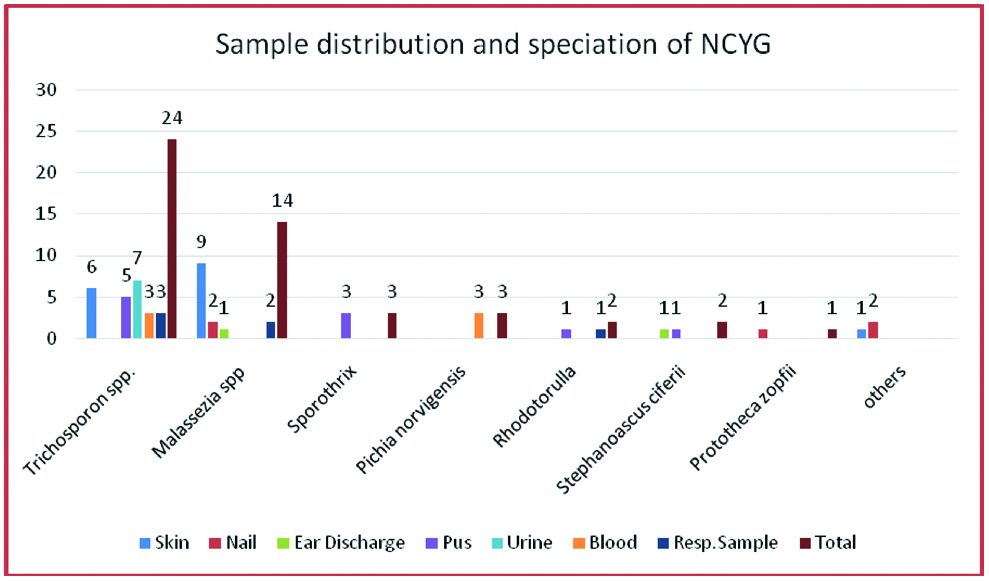
Further speciation of Tricosporon showed Trichosporonasahi 12, Tricosporonmucoides five, Trichosporoninkin three, and others four. Of these six were from skin samples, seven from urine, five from pus and soft tissue samples, three from blood and three from respiratory samples [Table/Fig-5].
Sample distribution and speciation of NCYG.
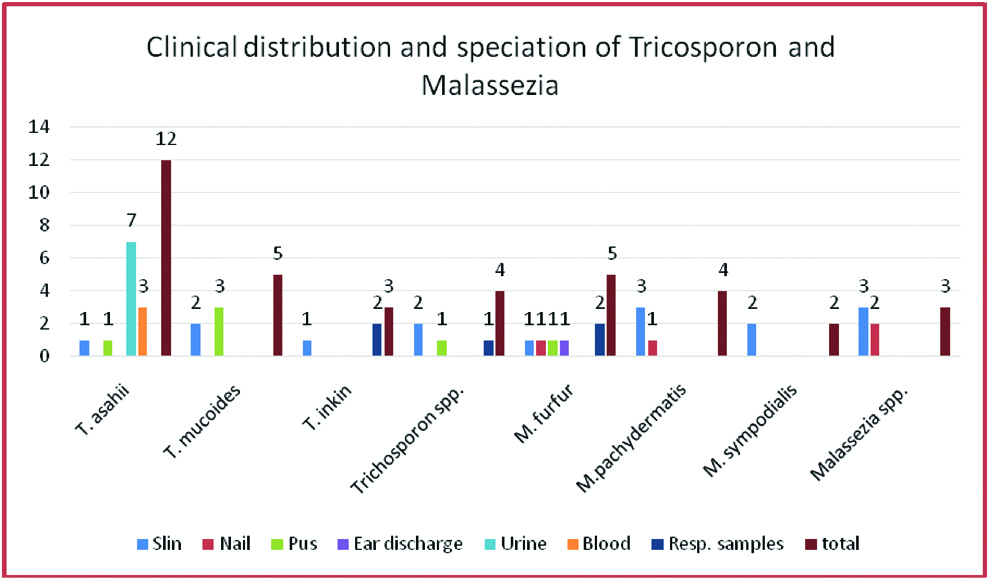
Malassezia speciation revealed M. Furfur five, M.pachydermatis four, M.sympodialis two and others three. Of these nine were from skin samples, two from nail, two from respiratory samples and 1 from ear discharge. The sample wise distribution of isolates is shown in [Table/Fig-6].
Clinical distribution and speciation of Tricosporon and Malassezia.
The male to female distribution of NCYG was 32:21 i.e., 1.5:1. Clinically isolates were from 33 MCC. This included 16 skin, five nail, 10 pus and soft tissue and two ear discharge. Among the rest, seven were from UTI, six were from BSI and 6 were from DSI. The DSI were pleural fluid, broncho-alveolar lavage and sputum.
Discussion
In recent years infections caused by NCYG among the immunocompetent patients are being reported. High mortality rate has been reported from leukaemia patients. Among the yeast-like fungal pathogens Candida are the most predominant. They accounted for 85.47% of these isolates in the present study. The disease spectrum, pathogenicity, diagnostic methods and speciation of which have been well-researched and published. Although Non-albicans Candida are the most common fungal yeast-like pathogens encountered in clinical specimen, infections due to Trichosporonspp. are increasing [4,5]. Isolation of genera other than Candida requires more laboratory tests and time for their identification. The occurrence of these other genera in the present study was 13.74%. Therefore, they should not be ignored.
Sporothrix schenckii was isolated in three cases of skin and subcutaneous infection from hand. This a common site of isolation [6]. Pichia is a telomorph. The anamorphs of some Pichia species are Candida species Candida inconspicua and Candida norvigensis belong to fluconazole-resistant emerging species that are more frequently isolated from invasive infections. In addition to this increasing concern, these two species are difficult to identify and differentiate by routine conventional methods [7]. There were three isolates of Pichia norvigensis, all isolated from blood sample and identified by VITEK 2C system in the present study.
Previously considered non-pathogenic, Rhodotorula spp. have emerged as opportunistic pathogens that have the ability to colonise and infect susceptible patients. They are known to cause nail and skin infections and also fungemia in Central venous catheterised patients [8]. Two Rhodotourula glutinis isolated in this study were 1 each from respiratory and wound sample [Table/Fig-5].
In previous studies Stephanoascus Ciferrii has been isolated previously from blood, wound swab, pus, and aural discharge and nail sample [9]. In this study there were two isolates of Stephanoascus Ciferrii of which one isolate was from pus and other one was from ear discharge from immunocompetent patients.
Trichosporonasahii is an emerging fungal pathogen seen particularly in immunologically compromised patients requiring antifungal susceptibility testing to start effective treatment. In the present study clinical features of disseminated infections includes; septic shock, renal failure and cutaneous lesion.
The commonest genus in this study among NCYG was Trichosporon. It was 46.15% (24/52). Trichosporon belongs to class Basidiomycetes with unique morphological characters of budding yeast cell and true mycelium that disarticulate to form arthroconidia [2,4]. Previously only one species T.beiglii was reported as pathogenic. Now among the major human pathogens are-T. asahi, T.asteroides, T.mucoides, T.inkin, T.ovoides and T.Cutanei [10]. They are inhabitants of soil and can be a part of normal skin flora. They are responsible for superficial skin infections including white piedra. They may be responsible for systemic infections in patients with haematological malignancies or patients on steroids. These species are increasingly emerging as opportunistic pathogens mainly in immunosuppressed patients. Trichosporon may also be confused with Geotrichumsp as both produce arthroconidia [4]. They can be differentiated on basis of urease test and corn meal test. Further the isolates in present study were tested with VITEK ID system. By this T.asahii, T.mucoides and T.inkin were identified [Table/Fig-6].
T.asahii was the most common Trichosporon spp. isolated in 50%, 12 out of 24 isolates. This has been reported in other studies from India, Japan, Turkey and Brazil [4,11-13]. T.mucoides was second most common, 20.83%, five out of 24. T.inkin was isolated in 12.50%, three out of 24. Others were 16.67%, four out of 24. Trichosporon spp. are associated with blood stream infections and Nosocomial UTI [14,15]. In the present study Trichosporon was isolated from wide range of clinical specimen. Urinary tract infection was the most common, followed my mucocutaneous infections. Like our study Trichosporon spp. have been isolated from pus, soft tissue and respiratory specimen [4,16] [Table/Fig-6] Though opportunistic they have also been reported from immunocompetent too. Risk factors such as prolonged multiple antimicrobials, indwelling catheters and co-morbidities such as anaemia and hypoalbuminemia may be contributory [17]. In the present study six isolates were from skin and nail samples, seven from urine, five from pus and soft tissue, three from blood and three from respiratory samples.
Susceptibility testing for Trichosporon: There are no standard guidelines for interpretative criteria for minimum inhibitory concentration testing. Some workers have used the criteria for Candida species for reference. These findings suggest poor susceptibility to Amphotericin B while azoles have shown good potency against T.asahii isolates. Though emergence of resistance has been reported from different parts of the world, it has not been reported from India [4,15].
Malassezia is a lipid dependent yeast-like fungus also belonging to Class basideomycetes. The genus has at least seven species M.furfur, M.globosa, M.sympodialis, M. obtuse, M.pachydermatis, M. slooffiae to name a few. Direct microscopy of specimen exhibits the characteristic appearance of “spaggetti and Meatballs” [18]. Speciation was done on gross morphology, microscopy, urease test, lipophilicity and by automated system VITEK using YST ID cards. A total of 14 isolates were obtained. Male preponderance was seen similar to other studies [18,19]. M.furfur was the commonest five isolates, M.pachydermatis was second, four isolates, M.sympodialis two and others three. The isolates were most commonly from skin specimen followed by nail, respiratory and ear discharge. Malassezia can now be added to a growing list of normal skin flora organisms of low virulence that may cause mild recurrent skin infections and systemic infection in susceptible host [19,20]. Thus, Trichosporon and Malassezia accounts for 73.08% (38 out of 52) of the NCYG in this study [Table/Fig-5,6].
In previous studies, Stephanoascus ciferii has been isolated previously from blood. Wound swab, pus, and aural discharge and nail sample [9]. In this study there were two isolates of Stephanoascus ciferii of which 1 isolate were from pus and other one was from ear discharge from immunocompetent patients [Table/Fig-5].
In the present study we reported onychomycosis, an uncommon presentation of protothecosis. Among the rare presentations there are few cases of fungual protothecosis and as per the published reports this could be the first report from India which has been cultured proven and then confirmed by National Culture Collection by Pathogenic Fungi (NCCPF), PGI Chandigarh [21].
Conclusion
NCYG are emerging as important opportunistic fungal pathogens and are likely to be overlooked. They require high index of suspicion and clinical correlation. The study highlights the presence of these genera in clinical samples which may have therapeutic implications. Their significance and management would require case to case correlation by the clinical microbiologist and clinician and consequently clinicians need to have increased awareness of these fungal infections. Therefore, it is necessary to identify these genera. Automated identification systems for yeast-like genera could be of great use towards this end.