Luminal A, Luminal B, human epidermal growth factor receptor 2 (HER2) overexpressing, basal-like, and normal-like are five distinct subtypes of breast cancer with different clinical outcomes [5].
The majority of Basal Like Breast Cancer (BLBCs) are characterised by the expression of basal cytokeratins (CKs) 5/6 and 17, Epidermal Growth Factor Receptor (EGFR), c-kit, and Vascular Endothelial Growth Factor (VEGF) and lack Estrogen Receptor (ER), Progesterone Receptor (PR), and HER2 protein overexpression [6,7].
The first step for glucose metabolism is the transport of glucose across the plasma membrane which is mediated by facilitative Glucose Transporter Proteins (GLUTs) that contain fourteen members [8,9]. GLUT-1, also named facilitates glucose transporter member 1 (SLC2A1) [10], is involved in glucose uptake in the basic state and is broadly expressed in the body tissues. GLUT-1 is elevated in almost all human cancers including brain, breast, head and neck, bladder, renal, colorectal, lung and ovarian cancers [9].
The aim of this study was to clarify the Immunohistochemical (IHC) expression of GLUT-1 and to examine any associations with pathological, clinical and survival data to help in assessing patient’s prognosis using 79 breast carcinoma patients.
Materials and Methods
This was a random retrospective study that included 79 specimens of breast invasive duct carcinoma retrieved from the archival cases of Pathology Department, Faculty of medicine, Menoufia University, Egypt, spanning the period between January 2010 and December 2017. The clinicopathological data were obtained from the patients’ sheets and TNM staging system (2010) is used for staging of the tumour according to size into T1, T2, T3 and T4 and to nodal status into Nx, N0, N1, N2 and N3 [11].
Immunohistochemistry
Paraffin-embedded tissue sections (5 μm) were deparaffinized in xylene and rehydrated. The sections were treated with 10 μM citrate buffer, pH 6.0, at 96°C for 10-20 minutes followed by 10 mL Tris-EDTA for 10-20 minutes. Endogenous peroxidase was blocked with peroxidase-blocking reagent (cat. #TP-015-HD) (Lab Vision Cooperation 46360 Fermont Blvd. Fermont, CA 94538-6406, USA, California) using GLUT-1 rabbit polyclonal antibody, {Thermo Fisher Scientific Anatomical Pathology (Fremont, CA)} with a dilution of 1:200. A positive reaction was revealed using the streptavidin-biotin-peroxidase technique (cat. #TP-015-HD) (Lab Vision Cooperation 46360 Fermont Blvd. Fermont, CA 94538-6406, USA) with chromogen DAB. The sections were counterstained with Mayer’s haematoxylin (Bio Genex, cat. No. 94583) for 30-60 seconds to stain nuclei then sections were washed in tap water for five minutes.
Red blood cells within tissue sections from a capillary haemangioma case were used as positive controls for GLUT-1. Also intravascular RBCs in the studied slides were used as internal control and negative controls were made by substituting the primary antibodies with non-immune serum.
Interpretation of Immunostaining Results of GLUT-1
Positivity for GLUT-1 was defined as detectable membranous staining in tumour cells. GLUT-1 immunostaining was quantified by grading the proportion of cells that were GLUT-positive. The grading system was as follows: absence of immunoreactive cells =negative; less than 10% of immunoreactive cells=1+; 10% to 50% of immunoreactive tumour cells=2+; and greater than 50% of immunoreactive cells=3+. For statistical analyses, each tissue section was classified as GLUT-1 positive or negative [12].
Overall Survival Data
By revising the patient’s files for breast carcinoma cases ranged from 2010 to 2017, overall survival time was available for all patients (100%).
Statistical Analysis
The statistical analysis was conducted using SPSS “Statistical Package for the Social Sciences” program for windows, version 20, SPSS Inc., Chicago, Illinois, USA. Mann-Whitney (U) and Kruskal-Wallis tests were used to compare nonparametric data and the chi-square was used to assess the association between the clinicopathological parameters and GLUT-1 expression. p-value ≤0.05 was considered to indicate statistical significance in all tests.
Results
The clinicopathological characteristics of invasive duct carcinoma cases are shown in [Table/Fig-1].
Clinicopathological data of the studied invasive duct carcinoma cases.
| Variable | n (%) |
|---|
| Age (years) |
| Mean±SD | 48.152±12.136 |
| Median | 48 |
| Range | 27-83 |
| Family history |
| Positive | 62 (78%) |
| Negative | 17 (22%) |
| Menopausal status: | |
| Premenopausal | 50 (63%) |
| Post menopausal | 29 (37%) |
| T stage (75 cases) |
| T1 | 11 (14%) |
| T2 | 39 (49%) |
| T3 | 10 (13%) |
| T4 | 15 (24%) |
| Nodal stage |
| Nx | 17 (22%) |
| N0 | 16 (20%) |
| N1 | 8 (10%) |
| N2 | 16 (20%) |
| N3 | 22 (28%) |
| Multicentricity |
| Present | 11 (14%) |
| Absent | 68 (86%) |
| Vascular invasion |
| Present | 7 (9%) |
| Absent | 72 (91%) |
| Perineural invasion |
| Present | 5 (6%) |
| Absent | 74 (94%) |
| Stage grouping |
| I | 5 (6%) |
| II | 14 (18%) |
| III | 50 (63%) |
| IV | 10 (13%) |
| Hormonal status |
| ER+, PR+ and Her2 neu+ | 12 (15%) |
| ER-, PR- and Her2 neu+ | 8 (10%) |
| Triple negative | 17 (22%) |
| ER+, PR+ and Her2 neu- | 32 (40%) |
| ER+, PR- and Her2 neu- | 6 (8%) |
| ER-, PR+ and Her2 neu+ | 0 (0%) |
| ER-, PR+ and Her2 neu- | 0 (0%) |
| ER+, PR- and Her2 neu+ | 4 (5%) |
| Grade |
| I | 6 (8%) |
| II | 22 (28%) |
| III | 51 (64%) |
| Extracapsular nodal invasion |
| Present | 5 (6%) |
| Absent | 74 (94%) |
| Metastasis |
| Present | 10 (13%) |
| Absent | 69 (87%) |
n: Number; SD: Standard deviation; M:F: Male to female
Immunohistochemical Results of GLUT-1 in Normal Breast Tissue
Some normal and hyperplastic mammary epithelial cells in tumour-free areas were GLUT-1-positive; however, the intensity of staining in these cells was lower than in cancer cells. GLUT-1-positive normal mammary cells exhibited a diffuse cytoplasmic staining, unlike the cancer cells, and their cell membranes were GLUT-1 negative.
Immunohistochemical Results of GLUT-1 in insitu Ductal Carcinoma
Cells of in-situ ductal carcinoma present in the studied carcinoma cases showed membranous staining similar to that of cancer cells. Immunohistochemical results of GLUT-1 in the studied invasive duct carcinoma cases are shown in [Table/Fig-2,3]. The relationship of GLUT-1 expression in invasive duct carcinoma cases and the studied clinicopathological parameters are shown [Table/Fig-4,5]. There was a highly statistical significant association between positive GLUT-1 expression and advanced nodal stage (p=0.001) and advanced T stage (p=0.000). Furthermore, there was a highly statistical significant association between positive GLUT-1 expression and poor degree of differentiation (Grade) (p=0.000). Moreover, there was a statistical significant association between high GLUT-1 positivity (+3) and advanced stage grouping (III and IV) (p=0.018). Also, there is a trend of significance between GLUT-1 expression and hormonal status as 94.1% of triple negative cases showed positive GLUT-1 expression (p=0.078).
Immunohistochemical results of GLUT-1 in the studied invasive duct carcinoma cases.
| Variables | n (%) |
|---|
| GLUT-1 expression | |
| • Positive | 63 (80%) |
| • Negative | 16 (20%) |
| Positive GLUT-1 expression | |
| • +1 | 8 (10%) |
| • +2 | 28 (35%) |
| • +3 | 27 (55%) |
a) Positive membranous GLUT-1 expression in a case of invasive duct carcinoma (IDC) G III (IHC X 200); b) Positive membranous GLUT-1 expression in a case of IDC G III with positivity in intravascular RBCs as internal control (IHC X 100); c) Positive membranous GLUT-1 expression in a case of IDC G II (IHC X 200); d) Positive membranous GLUT-1 expression in a case of IDC G I (IHC X 200); e) Positive membranous GLUT-1 expression in Insitue ductal carcinoma (IHC X 200) and f) Positive cytoplasmic GLUT-1 expression in normal breast lobules (IHC X 100)

The relationship of GLUT-1 expression in invasive duct carcinoma cases and the studied clinicopathological parameters.
| P value | Test | GLUT 1 expression | Variables |
|---|
| Positive | Negative |
|---|
| Age | 58.8±14.1 | 55.1±13.4 | U=0.254 | 0.604 |
| Family history |
| Positive | 3 (17.6%) | 14 (82.4%) | χ2=0.091 | 0.763 |
| Negative | 13 (21%) | 49 (79%) |
| Menopausal status |
| Premenopausal | 11 (22%) | 39 (78%) | χ2=0.257 | 0.612 |
| Post menopausal | 5 (17.2%) | 24 (82.8%) |
| T stage (75 cases) |
| T1 | 5 (45.5%) | 6 (54.5%) | χ2=5.605 | 0.231 |
| T2 | 7 (17.9%) | 32 (82.1%) |
| T3 | 1 (10%) | 9 (90%) |
| T4 | 2 (13.3%) | 13 (86.7%) |
| Nodal status |
| Nx | 3 (17.6%) | 14 (82.4%) | χ2=18.78 | 0.001** |
| N0 | 9 (56.3%) | 7 (43.8%) |
| N1 | 2 (25%) | 6 (75%) |
| N2 | 0 (0%) | 16 (100%) |
| N3 | 2 (9.1%) | 20 (90.9%) |
| Multicentricity |
| Present | 1 (9.1%) | 10 (90.9%) | χ2=0.986 | 0.321 |
| Absent | 15 (22.1%) | 53 (77.9%) |
| Vascular invasion |
| Present | 0 (0%) | 7 (100%) | χ2=1.951 | 0.163 |
| Absent | 16 (22.1%) | 56 (77.8%) |
| Perineural invasion |
| Present | 0 (0%) | 5 (100%) | χ2=1.356 | 0.244 |
| Absent | 16 (21.6%) | 58 (78.4%) |
| Stage grouping |
| I | 4 (80%) | 1 (20%) | χ2=20.128 | 0.000** |
| II | 6 (42.9%) | 8 (57.1%) |
| III | 4 (8%) | 46 (92%) |
| IV | 2 (20%) | 8 (80%) |
| Hormonal status |
| ER+, PR+ and Her2 neu+ | 5 (71.7%) | 7 (58.3%) | χ2=9.904 | 0.078 |
| ER-, PR- and Her2 neu+ | 0 (0%) | 8 (100%) |
| Triple negative | 1 (5.9%) | 16 (94.1%) |
| ER+, PR+ and Her2 neu- | 7 (21.9%) | 35 (78.1%) |
| ER+, PR- and Her2 neu- | 1 (16.7%) | 5 (83.3%) |
| ER+, PR- and Her2 neu+ | 2 (50%) | 2 (50%) |
| Grade |
| I | 5 (83.3%) | 1 (16.7%) | χ2=16.037 | 0.000** |
| II | 3 (13.6%) | 19 (86.4%) |
| III | 8 (15.7%) | 43 (84.3%) |
| Extracapsular nodal invasion |
| Present | 0 (0%) | 5 (100%) | χ2=1.356 | 0.244 |
| Absent | 16 (21.6%) | 58 (78.4%) |
| Metastasis |
| Present | 2 (20%) | 8 (80%) | χ2=0.000 | 0.983 |
| Absent | 14 (20.3%) | 55 (79.7%) |
n: Number; SD: Standard deviation; **: Highly-significant; χ2: Chi square; U: Mann-Whitney test; %: Percent
The relationship of GLUT-1 positive expression in invasive duct carcinoma cases and the studied clinicopathological parameters.
| Variables | Positive GLUT-1 expression | Test | p-value |
|---|
| +1 | +2 | +3 |
|---|
| Age | 45.2±11.1 | 46.1±10.2 | 48.3±12.4 | u=1.286 | 0.526 |
| Family history |
| Positive | 8 (16.3%) | 19 (38.8%) | 22 (44.9%) | χ2=4.094 | 0.129 |
| Negative | 0 (0%) | 9 (64.3%) | 5 (35.7%) |
| Menopausal status |
| Premenopausal | 5 (12.8%) | 16 (41%) | 18 (46.2%) | χ2=0.530 | 0.767 |
| Post menopausal | 3 (12.5%) | 12 (50%) | 9 (37.5%) |
| T stage (75 cases) |
| T1 | 3 (50%) | 2 (33.3%) | 1 (16.7%) | χ2=22.403 | 0.004** |
| T2 | 4 (12.5%) | 18 (56.3%) | 10 (31.3%) |
| T3 | 0 (0%) | 6 (66.7%) | 3 (33.3%) |
| T4 | 1 (7.7%) | 1 (7.7%) | 11 (84.6%) |
| Nodal status |
| Nx | 0 (0%) | 1 (7.1%) | 13 (92.9%) | χ2=32.588 | 0.000** |
| N0 | 2 (28.6%) | 5 (71.4%) | 0 (0%) |
| N1 | 3 (50%) | 3 (50%) | 0 (0%) |
| N2 | 2 (12.5%) | 10 (62.5%) | 4 (25%) |
| N3 | 1 (5%) | 9 (45%) | 10 (50%) |
| Multicentricity |
| Present | 0 (0%) | 3 (30%) | 7 (70%) | χ2=4.111 | 0.128 |
| Absent | 8 (15.1%) | 25 (47.2%) | 20 (37.7%) |
| Vascular invasion |
| Present | 0 (0%) | 4 (57.1%) | 3 (42.9%) | χ2=1.286 | 0.526 |
| Absent | 8 (14.3%) | 24 (42.9%) | 24 (42.9%) |
| Perineural invasion |
| Present | 0 (0%) | 3 (50%) | 2 (40%) | χ2=0.996 | 0.608 |
| Absent | 8 (13.8%) | 25 (43.1%) | 25 (43.1%) |
| Stage grouping |
| I | 1 (100%) | 0 (0%) | 0 (0%) | χ2=15.249 | 0.018* |
| II | 2 (25%) | 6 (75%) | 0 (0%) |
| III | 5 (10.9%) | 19 (41.3%) | 22 (47.8%) |
| IV | 0 (0%) | 3 (37.5%) | 5 (62.5%) |
| Hormonal status |
| ER+, PR+ and Her2 neu+ | 1 (14.3%) | 1 (14.3%) | 5 (71.4%) | χ2=7.551 | 0.673 |
| ER-, PR- and Her2 neu+ | 1 (12.5%) | 3 (37.5%) | 4 (50%) |
| Triple negative | 2 (12.5%) | 7 (43.8%) | 7 (43.8%) |
| ER+, PR+ and Her2 neu- | 3 (12%) | 13 (52%) | 9 (36%) |
| ER+, PR- and Her2 neu- | 0 (0%) | 3 (60%) | 2 (40%) |
| ER+, PR- and Her2 neu+ | 1 (50%) | 1 (50%) | 0 (0%) |
| Grade |
| I | 0 (0%) | 1 (100%) | 0 (0%) | χ2=7.206 | 0.125 |
| II | 4 (21.1%) | 11 (57.9%) | 4 (21.1%) |
| III | 4 (9.3%) | 16 (37.2%) | 23 (53.5%) |
| Extracapsular nodal invasion |
| Present | 1 (20%) | 2 (40%) | 2 (40%) | χ2=0.263 | 0.877 |
| Absent | 7 (12.1%) | 26 (44.8%) | 25 (43.1%) |
| Metastasis |
| Present | 0 (0%) | 3 (37.5%) | 5 (62.5%) | χ2=2.088 | 0.352 |
| Absent | 8 (14.5%) | 25 (45.5%) | 22 (40%) |
SD: Standard deviation; *: significant; **: highly significant; χ2: Chi square; U: Mann-Whitney test; %: Percent
Overall Survival
When revising the patients’ files for breast carcinoma overall survival time was available for all (100%) patients. The range of survival time was 1 to 105 months with 21.01±14.477 as mean±SD of months and a median of 20 months.
Univariate Survival Analysis for Breast Carcinoma Cases
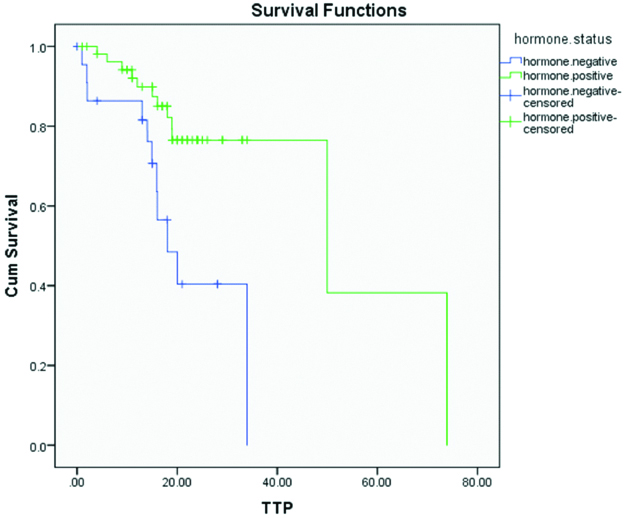
Univariate survival analysis revealed the bad prognostic impact of negative hormonal status (p-value=0.002) on patient outcome [Table/Fig-6,7]:
Univariate survival analysis for breast carcinoma cases.
| Variables | Mean | Median | p-value |
|---|
| Estimate | 95% CI | Estimate | 95% CI |
|---|
| Lower | Upper | Lower | Upper |
|---|
| GLUT 1 | - | - | - | - | - | - | 0.01 |
| Tumour size |
| T1 and T2 | 44.57 | 30.83 | 56.30 | 49.97 | 7.72 | 92.21 | 0.79 |
| T3 and T4 | 28.54 | 23.90 | 33.27 | 33.91 | - | - |
| Overall | 41.62 | 28.46 | 54.78 | 49.91 | 22.85 | 77.09 |
| Nodal status |
| Positive | 39.65 | 26.89 | 52.42 | 33.97 | 11.46 | 56.48 | 0.19 |
| Negative | 26.77 | 24.02 | 29.53 | - | - | - |
| Overall | 40.90 | 28.11 | 53.68 | 33.97 | 12.52 | 55.42 |
| Stage | |
| 1 and 2 | 27.02 | 24.52 | 29.51 | - | - | - | 0.09 |
| 3 and 4 | 38.86 | 26.27 | 51.46 | 33.97 | 11.29 | 56.65 |
| Overall | 40.90 | 28.11 | 53.68 | 33.97 | 12.52 | 55.42 |
| Hormonal status |
| Negative | 21.48 | 15.83 | 27.12 | 18.07 | 11.85 | 24.28 | 0.002** |
| Positive | 50.60 | 35.82 | 65.37 | 49.97 | 6.71 | 93.23 |
| Overall | 40.90 | 28.11 | 53.68 | 33.97 | 12.52 | 55.42 |
| Her 2 neu |
| Positive | 42.21 | 26.18 | 58.24 | 49.97 | 5.00 | 94.93 | 0.87 |
| Negative | 27.26 | 24.00 | 30.53 | 33.97 | - | - |
| Overall | 40.90 | 28.11 | 53.68 | 33.97 | 12.52 | 55.42 |
| Grade |
| 1 and 2 | 21.00 | 19.39 | 22.61 | - | - | - | 0.61 |
| 3 and 4 | 40.67 | 27.81 | 53.52 | 33.97 | 12.46 | 55.48 |
| Overall | 40.90 | 28.11 | 53.68 | 33.97 | 12.52 | 55.42 |
| Vascular invasion |
| Present | 22.82 | 20.72 | 24.91 | - | - | - | 0.40 |
| Absent | 40.28 | 27.54 | 53.02 | 33.97 | 12.36 | 55.58 |
| Overall | 40.90 | 28.11 | 53.68 | 33.97 | 12.52 | 55.42 |
| Perineural invasion |
| Present | 22.02 | 18.83 | 225.21 | - | - | - | 0.74 |
| Absent | 40.79 | 27.92 | 53.66 | 33.97 | 12.47 | 55.47 |
| Overall | 40.90 | 28.11 | 53.68 | 33.97 | 12.52 | 55.42 |
N.B: No descriptive statistics were computed for GLUT because all GLUT negative cases were censored to progression (No GLUT negative cases were progressed)
**: Highly significant; CI: Confidence interval
Kaplan-Meier overall survival for breast carcinoma patients with different hormonal status p<0.002 “highly significant”).
Discussion
Prevalence of GLUT-1 membranous expression in breast carcinoma cases was 71.8% [13] and range from 42% to 90% [14] and this was near the results in the present study as we found GLUT-1 expression in 80 % of the studied cases. Moreover, the present results were higher than that reported for the same antibody by Kang SS et al., (47%). These differences might have been related to differences in patient populations or tumour types.
Some normal and hyperplastic mammary epithelial cells in tumour-free areas were GLUT-1-positive; however, the intensity of staining in these cells was lower than in cancer cells. GLUT-1-positive normal mammary cells exhibited a diffuse cytoplasmic staining, unlike the cancer cells, and their cell membranes were GLUT-1 negative and this result agreed with Kuo SJ et al., [13]. However, this disagreed with Alo PL et al., who reported that 36% of typical/atypical hyperplastic breast tissue expressed GLUT-1 and normal adjacent tissues were positive in 31% of their cases [15].
Absence of membranous GLUT-1 expression in normal and hyperplastic breast tissue and its presence in in-situ ductal carcinoma and ductal carcinoma cases might clarify that GLUT-1 might have a role in early transformation process [15,16].
In the present study there was a statistical significant association between GLUT-1 positivity and poor prognostic factors including advanced nodal stage, advanced T stage, poor degree of differentiation (Grade) and advanced stage grouping (III and IV). These results agreed with Alo PL et al., who found that GLUT-1 expression was increased in poorly differentiated breast carcinoma and associated with high proliferative activity, increased invasiveness, and aggressive behaviour [15]. Moreover these results agreed also with Kawamura T et al., who reported that overexpression of GLUT-1 has been described in various malignant tumours and was associated with enhanced tumour aggressiveness and poor outcome [17].
Also the present study agreed with other studies that reported that GLUT-1 was also a prognostic molecular biomarker for patients with colorectal cancer liver metastasis [18].
These results could be explained as GLUT-1 could promote cell proliferation, migration and invasion by regulating epidermal growth factor receptor and integrin signaling in triple-negative breast cancer cells. Also, GLUT-1 was one of number of proteins acting through the Hypoxia-Inducible Factor 1 (HIF-1) pathway, which allowed tumour cells to survive the harsh tumour microenvironment and lead to resistance to radiotherapy and chemotherapy and is associated with a more aggressive phenotype with an increased propensity for metastases [19].
Inhibition of GLUT-1 might be used as an effective method for curing chemo-resistant breast cancer patients. As blocking of GLUT-1 can effectively decrease intracellular glucose level and induce energy stress in malignant cells, which initiates the activation of Adenosine Monophosphate Activated Protein Kinase (AMPK). AMPK might induce phosphorylation of tuberous sclerosis complex that increases its ability to suppress mammalian target of rapamycin (mTOR) activity, that could enhance the breast cancer cells death probably through activating AMPK and inhibiting Motor [20].
Furthermore, there was a trend of significance between GLUT-1 expression and hormonal status as 94.1% of triple negative cases showed positive GLUT-1 expression. These results agreed with Kang SS et al., who found that GLUT-1 expression correlated with negative ER and negative PR cases [15].
In the present study there was no statistically significant association between GLUT-1 expression and overall survival as no descriptive statistics were computed for GLUT-1 because all GLUT-1 negative cases were censored to progression and these results agreed with Kuo SJ et al., who found that there was no correlation between GLUT-1 expression and either recurrence or survival rate [13].
However, these results disagreed with Kang SS and Chun YK who found that GLUT-1 expression correlated with poor disease free survival. These differences might have been related to differences in patient populations or tumour types.
Limitation
The small number of invasive duct carcinoma cases as they were 79 cases only.
Conclusion
GLUT-1 is a poor prognostic marker in view of association between positive GLUT-1 expression and advanced nodal stage, advanced T stage, advanced stage grouping (III and IV) and poor degree of differentiation (Grade). Furthermore, inhibition of GLUT-1 might play a therapeutic role for triple negative breast cancer.
n: Number; SD: Standard deviation; M:F: Male to female