Herpes viruses are large, enveloped DNA viruses and Herpes simplex viruses are widespread in human population. They show a broad host range as they are able to survive in different types of cells and are also capable of infecting a wide range of animals. The spectrum of infections caused by them ranges from gingivostomatitis to keratoconjunctivitis, encephalitis, genital disease and infections in newborns [1]. Herpes simplex viruses are neurotropic and are an important cause of central nervous system infections. According to the western literature, Herpes Simplex Virus (HSV) is the most common cause of sporadic focal viral Central Nervous System (CNS) infection. To date, there is no evidence in contrary from India or elsewhere in the world [2,3]. The exact incidence of this disease is difficult to estimate as only the severe cases report to hospitals whereas the mild cases go unnoticed. In India, HSV encephalitis is usually underdiagnosed due to lack of facilities.
There are no clinical features specific for HSE as it mimics other viral encephalitis, focal infections and non-infectious processes. Although isolation of virus from brain biopsy tissue is considered the definitive laboratory diagnostic test for CNS infection due to HSV, collection of brain tissue for microbial diagnosis is a highly invasive procedure and is very uncommon in routine medical practice. Immunological analyses of CSF for the presence of specific antigens or antibodies to HSV and recovery of virus from this source has not yielded either sensitive or rapid results for the laboratory diagnosis of HSV related CNS disease [4]. There are only few published reports of molecular diagnosis of HSV encephalitis from India and none from Pondicherry and hence, present study was undertaken to evaluate immunological and molecular method for rapid diagnosis of HSE.
Materials and Methods
Present study was conducted in the Department of Microbiology in collaboration with the Department of Neurology, JIPMER, Puducherry over a period of 2 years. The study was approved by Institute research and ethics committees. CSF samples were collected by lumbar puncture from cases of clinically suspected viral encephalitis after obtaining written informed consent from the patient or guardian. The samples were transported on ice to the Microbiology laboratory and were stored at 40C for short-term and -800 C for long-term storage. Eight CSF samples from patients with confirmed CNS infections due to other causes and 12 samples from post-operative surgery cases were also collected as a part of control group. All the samples were tested by two methods: Nested Polymerase Chain Reaction (PCR) for detection of glycoprotein D gene for HSV and Dot-ELISA (Enzyme Linked Immuno-Sorbent Assay) for HSV antigen detection.
Nested Polymerase Chain Reaction for HSV
DNA from the samples was extracted using the commercial QIAmp Viral RNA Mini kit (QIAGEN, USA) according to the manufacturer’s instructions. The kit extracts both RNA and DNA present in the sample and are collected in fresh micro-centrifuge tube and subjected to PCR. The PCR performed was a nested multiplex PCR adapted from Kessler et al., 2000 with some modifications. The primers used targeted the glycoprotein D of Herpes simplex virus common to both HSV-1 and HSV-2. Primer details are as follows:
Round 1: Product size of 200bp. Forward (5’-TGCTCCTACAACAAGTC), Reverse (5’-CGGTGCTCCAGGATAAA). Round 2: Product size of 142bp. Forward (5’-ATCCGAACGCAGCCCCGCTG), Reverse (5’-TCTCCGTCCAGTCGTTTATCTTC). Primer mix 1 and 2 were prepared by mixing 96 μl of milli-Q water and 2 μl of forward primer of primer 1 or 2 and 2 μl of reverse primer of primer 1 or 2 respectively.
The reaction mixture for Round 1 was prepared as follows: The components added included 12.5μl of commercial Mastermix (Genei, Bangalore) per reaction. The concentration of primer mix was tried at 2 μl, 3 μl and 4 μl and 4μl was found to be optimal. The amount of template DNA added was tried at 2 μl and 3 μl per reaction, there was no difference between the two volumes and 2μl of template DNA was added per reaction. The volume of milli-Q water was adjusted to make a final reaction volume of 25 μl. PCR conditions used in Round 1 and 2 are summarised in [Table/Fig-1].
Summary of PCR conditions used for Round 1 &2 of Nested multiplex PCR.
| PCR conditions used for Round 1 | PCR conditions used for Round 2 |
|---|
| 1. 950 C for 10 minutes followed by 2. 40 cycles of:940 C for 30 seconds for denaturation 500 C for 20 seconds for annealing and 720 C for 30 seconds for extensionFinal extension at 720 C for 1 minuteThe expected product size - 200bp | 1. 950 C for 10 minutes followed by 2. 40 cycles of:940 C for 30 seconds for denaturation 620 C for 15 seconds for annealing and 720 C for 20 seconds for extensionFinal extension at 720 C for 1 minuteThe expected product size- 142bp |
Round 2: The reaction mixture for Round 2 was prepared in the same way as round 1 except for the fact that 2 μl of the amplicon of the Round 1 was added instead of extracted DNA. The amplified products at the end of second round were visualised using a 1.8% agarose gel with Ethidium Bromide where the amplified products were allowed to undergo electrophoresis at a constant voltage of 80 volts for 45 minutes. Ten microlitre of DNA and 2 microlitre of loading dye (Thermoscientific 6x gel loading buffer) and Tris Borate EDTA (TBE) buffer was used for running the gel electrophoresis. The bands were visualised under UV illumination and the product size was ascertained in comparison to a standard 100 bp DNA ladder used as a molecular weight marker. The samples showing a band at the region of 142 bp were considered positive. The results of gel electrophoresis are depicted in [Table/Fig-2]. With each run of PCR, a positive control (DNA extracted from tissue culture of HSV-1) and negative control (nuclease free water) were included. In order to rule out the presence of PCR inhibitors in the sample, all negative samples were retested after adding extracted DNA of tissue culture derived HSV to each sample.
Agarose gel electrophoresis of the amplified products of nested PCR for detection of glycoprotein D gene of HSV
M – 100 bp Ladder
Lane 1 – Positive control
Lane 2 – Negative control
Lanes 3 & 7 – Positive for glycoprotein D gene of HSV
Lanes 4,5,6 – Negative for glycoprotein D gene of HSV

DOT-ELISA for Detection of HSV Antigen
For performance of Dot-ELISA, plastic strips of size approximately 4.5 x 0.5cm were cut out. The nitrocellulose membrane was cut in small squares of size 0.5x 0.5 cm and attached to the end of plastic strip by DPX mount. The strips were then allowed to dry overnight and stored at room temperature till further use. Goat polyclonal antibodies against HSV-1 and mouse monoclonal antibodies specific HSV-1 glycoprotein E antigen were obtained from commercial sources (Pierce Biotechnology, Rockford IL 61105 USA through Genex India Biosciences, Chennai). The antigen for standardisation was prepared from a tissue culture extract of HSV grown in Vero cell lines.
A known HSV tissue culture fluid from where antigen was extracted by centrifugation, suspension in glycine buffer and sonication was used as positive control for Dot-ELISA procedure. On Nitrocellulose Membrane (NCM), 2 μl of polyclonal antibody was spotted, and then allowed to air dry for 5-15 minutes. The strips were immersed in 3% bovine serum albumin for 45 minutes to block the other areas of NCM. After blocking, the strips were washed thrice with Phosphate Buffered Saline (PBS) of pH 7.2 with 0.1 % tween 20. The washed strips were exposed to 5μl of antigen (centrifuged deposit of CSF samples) and kept at 370 C for 1 hour in a sterile glass petri dish which acted as moist chamber. The strips were then washed thrice with PBS with 0.1% tween 20. The strips were subsequently subjected to 5 μl of 1: 20 dilution of monoclonal antibody and incubated at 370C for 1 hour in a similar moist chamber. This was followed by three washes with PBS containing 0.1% tween 20. The HRP- conjugated anti-mouse monoclonal antibody was diluted in 1in 1000 and the washed strips were incubated in the diluted conjugate for one hour at 370C. This was followed by three washes with PBS containing 0.1% tween 20. The strips were then exposed to the Di-amino-benzidine substrate in diluted hydrogen peroxide for 15 minutes to half an hour. The reaction was stopped with distilled water. Development of a brown dot was taken as positive reaction.
Results
A total of 45 CSF samples were collected from patients >12 years admitted in JIPMER with clinically suspected Herpes simplex encephalitis during the two-year study period from August 2009 to June 2011. All the 45 CSF samples of the suspected HSE patients were tested by nested PCR and Dot-ELISA for presence of HSV.
After the second round of PCR, 4 of the 45 samples showed a band at region of 145bp and hence were considered positive for HSV DNA and are depicted in [Table/Fig-2]. The positive and negative controls were satisfactory. All the negative samples were tested for the presence of PCR inhibitors by spiking them with the extracted DNA of tissue culture derived HSV. One of the samples did not give any band even with addition of HSV DNA and hence, was excluded from the study. Hence positivity rate of PCR in the test group was calculated as 9.1% (4/44).
None of the samples tested by Dot-ELISA gave a positive result, including the 4 that were positive by PCR.
Twenty CSF samples from patients with confirmed CNS infections due to other causes and post-operative neurosurgery cases which were collected as part of control group were also tested by nested PCR and Dot-ELISA for HSV and were found to be negative. Since only 4 samples turned out to be positive for HSV DNA by PCR, a brief summary of the four cases are presented in [Table/Fig-3].
Summary of clinical presentation, investigations, management and outcome of 4 HSV PCR positive patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|
| Age and Sex | 35/M | 40/F | 24/F | 15/F |
| Presenting complaints | Fever, headache, altered sensorium | Fever, headache, altered sensorium & seizures | Fever, headache, altered sensorium & herpes labialis | Fever, headache, altered sensorium & seizures |
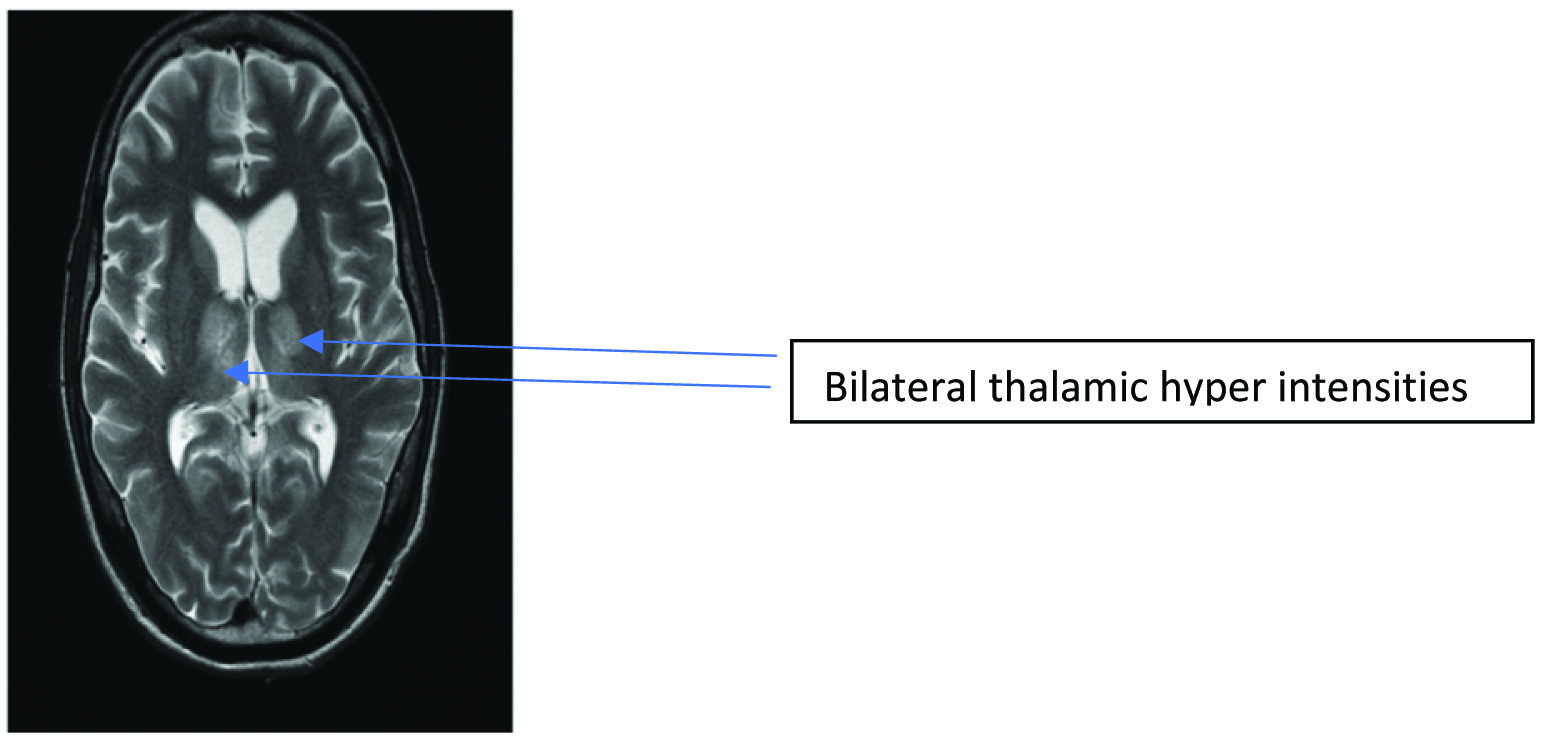
| Radiological abnormalities | MRI brain-involvement of thalamus & basal ganglia[Table/Fig-2] | CT brain- calcified granuloma in right parietal region | CT brain with i.v. contrast-multiple small hypodense areas in bilateral frontal lobes and left temporal lobe with minimal oedema | Contrast enhanced CT -communicating hydrocephalus |
| Cytochemical analysis of CSF | Cells -presentProtein-56mg/dl | Cells -presentProtein-483 mg/dl | Cells -presentProtein-70mg/dl | Cells -presentProtein-137mg/dl |
| Adenosine deaminase (ADA) levels | NA | 7IU/L | 28IU/L | 18IU/L |
| Day of PCR positivity after disease onset | Day 2 | Day10 | Day 3 | Day 7 |
| Duration of acyclovir | 14 days | 14 days | 14 days | 5 days |
| Outcome | Survived with sequelae | Survived with sequelae | Recovered without sequelae | Survived with sequelae |
Discussion
HSE is the commonest cause of sporadic viral encephalitis [5-7]. It causes a wide spectrum of illness ranging from asymptomatic to mild illness to severe necrotising encephalitis. Only symptomatic patients seek medical attention and hence, its incidence cannot be accurately determined. Diagnosis of HSE has come a long way and no longer relies upon brain biopsy followed by viral culture that was once used as the “gold standard” [8]. Other diagnostic approaches included antigen detection in CSF and demonstration of four-fold rise in titres of intrathecal antibodies. These invasive and cumbersome procedures have been replaced by detection of viral nucleic acid in CSF [9]. HSV DNA can be detected in CSF for up to 2 weeks from the time of disease onset and is an indicator of active viral infection [10]. Among the various methods used for diagnosis of HSE, PCR for HSV DNA in the CSF has emerged as standard reference test with sensitivity of 95-99% and specificity of 99-100% [5,9-11]. Another key advantage of PCR is that, unlike traditional methods, it retains its sensitivity even after a short course of acyclovir and hence empirical treatment can be started without compromising the laboratory diagnosis.
In the present study, 4 out of 45 CSF samples from clinically suspected cases of HSE were found to be positive by PCR. This indicates a positivity rate of 9.1%. Studies have shown that HSE accounts for 2-19% of all cases of viral encephalitis and 20-75% of all necrotising encephalitis [12]. And of these, a majority of cases i.e. about 90% are due to Herpes simplex type-1 virus. Recently molecular studies from India have shown that HSV accounts for 14-35% of all suspected viral encephalitis [13-15]. In previous studies, detection rates by PCR has been widely variable ranging from rates as low as 1% to 7% [4,6,10,16-19]. Madhavan HN et al., 1999 in a study done at Chennai on various clinical samples detected HSV DNA in 7 of the 20 CSF samples, recording a much higher positivity rate of 35 % in their study [15]. Rahman W et al., 2010 has detected HSV DNA by Real Time PCR in 7 of the 50 patients in North India giving a positivity rate of 14% [9]. Though the primers used in the present study could not differentiate between HSV-1 and HSV-2, it is probable that all the PCR positive are HSV-1 encephalitis as over 90% of the HSE is due to HSV-1 [16].
HSV antigen detection in CSF was also attempted in the present study to determine its utility in the diagnosis of HSE. In the present study, antigen detection by Dot-ELISA did not detect any positives in the CSF samples of patients with suspected HSE, including the 4 PCR positive patients and could not be statistically compared with PCR. The reason for poor sensitivity rate of the CSF samples by antigen detection could be that the positive control, being a culture isolate of the virus, would have a much higher load of the antigen than that would be present in the CSF sample, indicating the superior sensitivity of PCR in detecting lower quantities of the virus as found in clinical samples.
It is well-established that the signs and symptoms of HSE mimics that of other viral encephalitis. All of the four patients whose HSV PCR turned out to be positive presented with a similar clinical presentation of fever, altered sensorium and headache. Altered sensorium followed by seizures was the most common clinical manifestations of HSE patients according to Panagariya A et al., 2001 [20]. Behzad- Behbahani A et al., 2003 also did a comparison of various clinical symptoms with PCR positivity and found that alterations in the level of consciousness and lateralisation signs were significantly associated with HSV PCR positivity [21]. Radiological involvement of the infero-medial region of the temporal lobe is classically associated with HSE but lacks sensitivity and specificity. In the present study, only 1 of the 4 patients of the positive PCR group showed these features. Studies by Behzad- Behbahani A et al., 2003 found that evidence of temporal lobe involvement as shown in MRI in [Table/Fig-4] was significantly associated with PCR positivity. Pleunmpanupat P et al., 2009 concluded that the clinical presentation in HSE is similar to other viral encephalitis except for the radiological involvement of the temporal lobe [22].
MRI-T2 image showing bilateral thalamic hyperintensities, rarely seen in HSE.
Cytochemical changes in the CSF including presence of cells in CSF and elevated proteins have been used as screening criteria for CSF samples to increase the PCR positivity rate [17,23]. These cytochemical changes were seen in all 4 of the HSV PCR positive patients. Only 1 of the 4 patients of the positive PCR group showed these features. Studies by Behzad- Behbahani A et al., 2003 found a significant association of increased lymphocytes with PCR positivity [21].
A surprising finding in the present study was elevated CSF Adenosine Deaminase (ADA) levels (>10IU/L) in 2 of the 4 patients in the HSV PCR positive group which is usually seen in patients with tuberculous meningitis. Only in one study by Lopez-Gomez M et al., 2003 analysed the occurrence of elevated ADA levels in a case of PCR proven HSE [24]. The authors also highlighted the fact that the ADA levels in their patient returned to normal after a 14-day course of acyclovir. In the present study, 2 of the HSV PCR positive patients who had elevated ADA levels did not undergo serial analysis of the CSF and hence, the levels post-treatment were not available.
Mortality rates in HSE ranges from up to 70% in untreated patients [5,25,26] to 19-38% in treated patients [17,23]. Morbidity in patients even when treated with acyclovir could be as high as 61% with persistent neurological sequelae. All the 4 HSV PCR positive patients received acyclovir therapy. However, only one person was completely relieved of symptoms. Other 3 had persistent neurological sequelae, which underlines the severity of HSE, even in the presence of treatment with acyclovir.
Conclusion
Rapid diagnosis of HSE by molecular methods is helpful for early diagnosis and timely initiation of acyclovir therapy leading to a better clinical outcome. Nested PCR of the CSF is a useful method for diagnosis of HSE while antigen detection has little or no practical value.