Nearly 10-15 percent of Middle Cerebral Artery (MCA) infarctions may have cerebral oedema significant enough to cause raised intracranial tension and brain herniation, thus leading to progressive clinical worsening and death. Such MCA infarctions are termed as ‘malignant MCA infarctions (mMCAI) [1-3]. Malignant MCA infarcts are usually complete MCA infarcts because of the occlusion of distal Internal Carotid Artery (ICA) infarcts or MCA trunk occlusion. A complete MCA infarct, by virtue of its large size, is often accompanied with massive brain oedema resulting in progressive neurological deterioration, finally leading to transtentorial herniation causing brain death. Brain death usually occurs within 2-5 days [1]. Malignant MCA infarcts are highly fatal with various studies reporting the mortality rate from 40% to 100% [2,4,5]. Hacke W et al., reported 78% mortality among mMCAI patients despite best possible conservative management in the first month [2]. Most of the figures regarding mMCAI have emerged from the western studies and Indian data is scarce regarding the same. Recently, Rai V et al., reported 83% mortality rate at one year of follow up, in 36 patients managed on conservative treatment in a study [6].
Several studies worldwide have shown that DHC of mMCAI has resulted in better patient outcomes in comparison with the best conservative management. However, major benefit appears to be in terms of reduction in mortality rate only at the expense of functional disability of the survivors after the surgery [7]. Yang MH et al., in a recently published meta-analysis concluded that DHC led to significantly decreased mortality in mMCAI, though with a non-significant increase in proportion of patients who survived with major disability [8]. Respecting Indian data from AIIMS study, DHC in mMCAI had shown clear advantage in reducing the death rate with absolute risk reduction of 45% at one year, in comparison with the best medical management alone. The same study has also reported the improved functional outcome in surgical group with 20% of the patients (and 32% of the survivors) having good outcome (defined at mRS≤3) at one year, while none of the surviving patients on medical management achieved the same [6]. Although, the reduced mortality rates with DHC, as observed in this study are in line with the most of the western data available, however higher proportion of survivors among surgical group achieving good outcome (mRS≤3) as compared with medical group, is not in agreement with the most of the published data [7-9].
Based on the promising results of AIIMS study among Northern Indian patients regarding good functional outcome among survivors after DHC in mMCAI, we aimed to compare the outcomes of DHC and best medical management in similar patient population attending one of the largest tertiary referral centers in Southern India.
Materials and Methods
This was a prospective, non-randomised interventional comparative study in which the functional outcome of surgical and medical management groups was compared among the mMCAI patients. The study enrolled the patients attending the inpatient services of Emergency Medicine, Neurology department at Sri Venkateswara Institute of Medical Sciences (SVIMS) at Tirupati, a prominent tertiary referral center in Southern India. The study duration encompassed from November 2015 to October 2016. The study comprised of 60 patients having mMCAI out of which 20 underwent surgery and 40 received the best medical management alone. Institutional ethics committee approved the study protocol and a written informed consent was obtained from all patients (or their close relatives if patients could not give consent due to impaired consciousness or neurological deficit) enrolled in the study.
Patient Selection
All consecutively presenting patients with acute neurological signs and symptoms and showing infarct in more than 50% of MCA territory on CT, were assessed towards meeting the clinical inclusion criteria {National Institute of Health Stroke Scale (NIHSS) score ≥16 and decline in consciousness level according to NIHSS item 1a score of ≥1 point} for DHC as per the ‘2015 update of Korean clinical practice guideline for stroke recommendations for decompressive craniectomy in patients with mMCAI’ and if suitable, were prospectively enrolled [10]. Patient’s family members or attendants were informed about ‘survival with severe disability’ and ‘lack of benefit on quality of life’ as the potential outcomes of surgery. Performing DHC within 48 hours of the stroke onset was kept as mandatory criteria for the surgery group [10].
Exclusion Criteria
Some of the study subjects were excluded to reduce the risk of bias. Those patients, who died within 48 hours of the stroke onset and those with dilated and fixed pupils at the presentation, were excluded. Further, those with Glasgow Coma Scale (GCS) score < 6 and mRS ≥ 2, prior to the onset of presenting event, were excluded [6,11]. Those who were detected to have metabolic causes of impaired consciousness also met the exclusion criteria.
Comparative Group
Some of the patients could not undergo surgery despite meeting all the inclusion requirements. The reasons for the same were lack of written informed consent, timely unavailability of neurosurgeon, abnormal coagulation profile and severe co-morbidities posing a high risk to the surgery. These patients received the best medical management and formed the comparison group. Their treatment protocol included admission into intensive care units, optimization of the vital parameters, hyperosmolar therapy, oxygen support and mechanical ventilation, if needed. The outcomes in comparison group were compared with the outcomes of cases who underwent surgery.
Patient Evaluation
The clinical suspicion of stroke was confirmed by Non-contrast CT (NCCT) scan of brain. Detailed demographic variables were entered into case proformas along with the presenting clinical features, risk factors for stroke, blood pressure, GCS and NIHSS scores and imaging findings.
Treatment
DHC was done by creating a large fronto-parieto-temporal free bone flap and duroplasty, while brain tissue did not undergo any intervention. Free bone flap was placed in abdominal subcutaneous fat pocket. A subcutaneous drain was kept for 24 hours after the surgery and patients were kept in Intensive Care Unit (ICU). A postoperative CT scan was routinely done to rule out hematoma formation and see for adequate decompression. Details regarding the surgery including time of onset of symptoms to surgery, post-operative complications and duration of stay in ICU were noted.
Hospital Stay and Follow up
Details about the duration of hospital stay, complications and death during the stay were noted. Clinical assessment at the time of discharge was done using GCS, NIHSS and mRS scores and noted accordingly. Outcome after discharge was based on mRS scores assessed during follow up Out-Patient Department (OPD) visits or telephonically, whatever feasible at three and six months. Patients with mRS≤3 were considered to have good outcome.
Statistical Analysis
Statistical analysis was done using SPSS IBM version 16.0 (Chicago, IL). Continuous variables were compared using ‘Student’s t-test’ and categorical variables were compared using ‘Chi-square test’. Continuous and categorical variables were expressed as mean±standard deviation and percentages, respectively. Relative risk was analysed with 95% Confidence Interval (CI) and ‘Number Needed to Treat (NNT)’ was calculated for the cases. Survival analysis was done using Kaplan-Meier survival curve.
Split Data Analysis
Respecting ‘2015 updated Korean clinical practice guidelines for DHC in mMCA infarctions’ which mention level 1a and 1b recommendations for surgery in patients with mMCA infarctions with age ≤60 and those with >60 years, respectively, we also performed a split data analysis of those with more than 60 years forming one subgroup and those with 60 years or younger forming other subgroup. In addition to the combined data analysis, this age based subgroup analysis was done to observe any significant difference in mortality and disability outcomes in our patients in the two subgroups.
Results
A total of 70 patients with mMCAI during the study period were considered for enrolment, out of which 10 were excluded for the reasons mentioned above. So, the final study group comprised of 60 patients. Mean age was 53 years and 47(78%) were males. A total of 24(40%) patients were more than 60 years of age. Among the study group, 20(33%) patients underwent DHC while 40(67%) patients received the best medical management.
Baseline Characteristics of the Patients in Medical and Surgical Groups
Both the groups had similar distribution of most baseline variables (including gender, GCS and NIHSS scores) except age, frequency of aphasia and midline shift. Mean age of the patients undergoing surgery was significantly less than the medical management group (46.40±15.77 vs 56.95±13.578, p=0.01). Further, midline shift >5mm and presence of aphasia as a presenting symptom, were significantly more frequent in the surgical group (90% vs 50%; p=0.002 and 50% vs 23%; p=0.031, respectively). [Table/Fig-1] depicts the comparison among baseline characteristics of the patients in surgical and medical management groups.
Baseline characteristics of the patients in medical and surgical management groups.
| Patient Characteristics | TOTAL (n=60) | MedicalGroup (n=40) | SurgicalGroup (n=20) | Significance(p) |
|---|
| Age in years (SD) | 53.45 (14.973) | 56.95 (13.578) | 46.400 (15.779) | 0.010 |
| GCS at Admission (SD) | 9.33 (2.488) | 9.50 (2.651) | 9.00 (2.152) | 0.468 |
| GCS at Discharge (SD) | 9.733 (4.169) | 9.43 (4.408) | 10.35 (3.675) | 0.423 |
| NIHSS (SD) | 16.55 (4.924) | 17.23 (4.844) | 15.20 (4.927) | 0.134 |
| Male (%) | 47 (78.3) | 30 (75) | 17 (85) | 0.375 |
| Smoking (%) | 26 (43.3) | 14 (35) | 12 (60) | 0.065 |
| Dyslipidaemia (%) | 17 (28.30) | 9 (22.50) | 8 (40.00) | 0.156 |
| Hypertension (%) | 38 (63.30) | 25 (62.5) | 13 (65.0) | 0.850 |
| Diabetes mellitus (%) | 22 (36.70) | 18 (45.0) | 4 (20.0) | 0.058 |
| Aphasia (%) | 19 (31.70) | 9 (22.50) | 10 (50.0) | 0.031 |
| Large Artery Stroke (%) | 50 (83.30) | 33 (82.50) | 17 (85.0) | 0.806 |
| Cardio-embolic stroke (%) | 10 (16.70) | 7 (17.5) | 3 (15.0) | 0.806 |
| Metabolic abnormalities (%) | 6 (10.0) | 2 (5.0) | 4 (20.0) | 0.068 |
| Midline Shift >5 mm (%) | 38 (63.3) | 20 (50.0) | 18 (90) | 0.002 |
Continuous variables were compared using ‘Student’s t-test’ and categorical variables were compared using ‘Chi-square test’ for descriptive analysis; SD- Standard deviation; p-value in bold suggest a statistically significant difference between the two groups.
Cumulative Death Risk and Survival Analysis
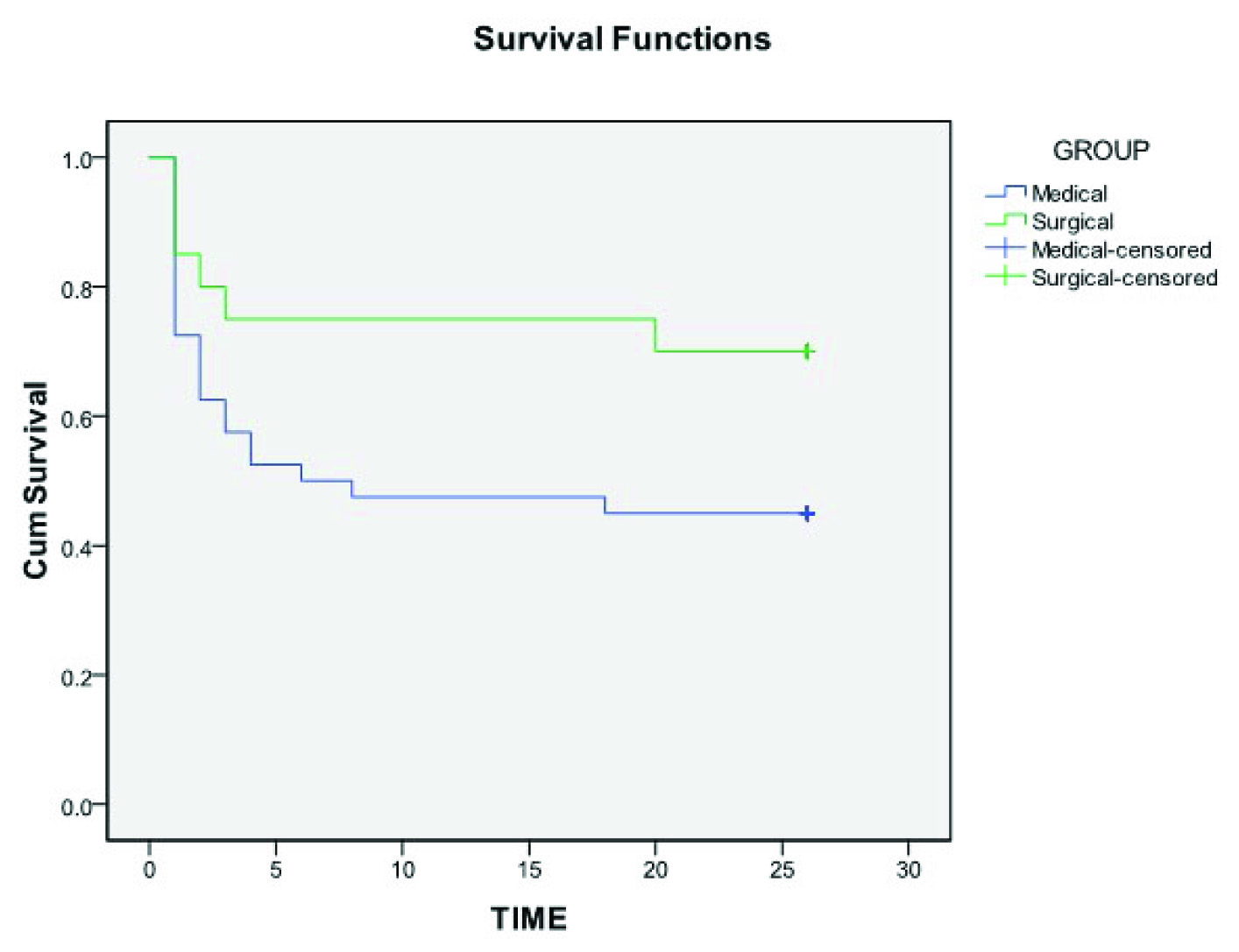
Higher mortality rate was observed in medical management group as compared with DHC group during the hospital stay, and at three months and six months follow up period. However, at any time during the course of follow up, the difference in mortality rates between the two groups did not reach statistical significance. Cumulative death rate at the end of six months in medical and surgical group was 55% and 30% respectively, with an Absolute Risk Reduction (ARR) of 25% and NNT of 4 [Table/Fig-2]. Kaplan meier Log rank test showed better cumulative survival probability in the surgical group, still, the difference between the two curves was not statistically significant (p=0.075) [Table/Fig-3].
Cumulative death risk in medical and surgical management groups.
| Medical Group (n=40) | Surgical Group (n=20) | ARR | RR | NNT |
|---|
| Discharge | 0.275 (11/40) | 0.15 (3/20) | 0.125 | 0.54 | 8.0 |
| 3 Months | 0.525 (21/40) | 0.25 (5/20) | 0.275 | 0.47 | 3.6 |
| 6 Months | 0.55 (22/40) | 0.30 (6/20) | 0.25 | 0.54 | 4.0 |
ARR: Absolute risk reduction; RR: Relative risk; NNT: Number needed to treat
Comparison of Kaplan meier survival curves among medical and surgical management groups.
Split Data Analysis of Death Risk and Survival Curves in Patients with Age ≤60 and >60 years
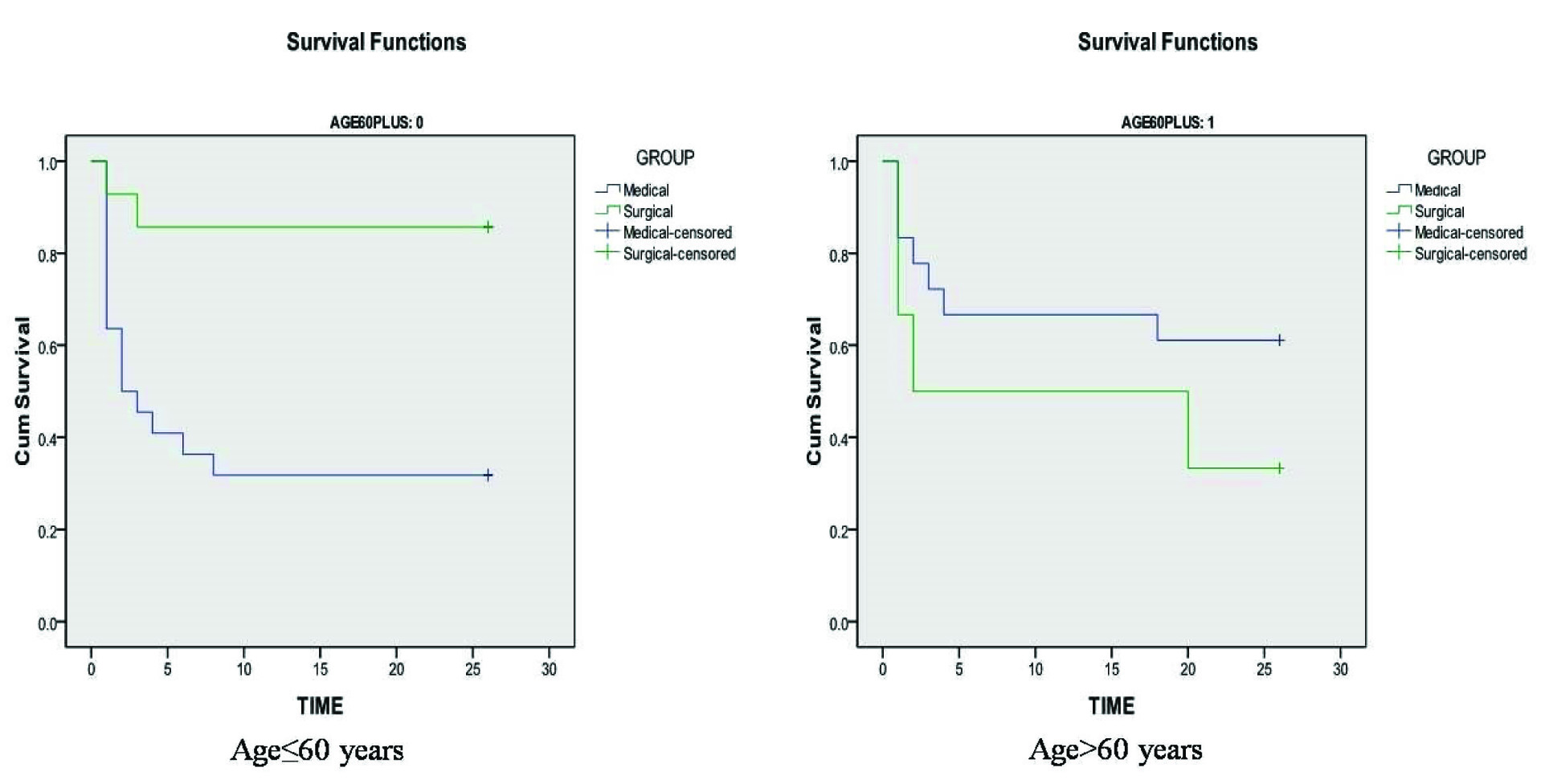
Patients with age ≤60 years who were treated with the medical management alone had a higher mortality rate after six months, in comparison to the surgical management group (68% vs 14%). This difference was statistically significant with p-value of 0.004 (OR=12.85, CI=2.244-73.635) [Table/Fig-4]. So, unlike the combined group analysis, where the higher mortality rate in medical management group was not statistically significant, comparison of younger patient population revealed significantly high mortality in the patients who did not undergo DHC. In contradiction, comparison of medical management and DHC groups among patients with more than 60 years of age, revealed higher death rate in surgical group (39% vs 67%) at six months follow up. Nevertheless, this difference was not statistically significant (p=0.248) [Table/Fig-4]. Survival analysis with Kaplan meier Log rank test also corroborated the above results by showing a significantly better cumulative survival probability in patients ≤ 60 years who underwent surgery (p=0.002) in comparison to the patients of same age subgroup who were treated with best medical management alone [Table/Fig-5]. On the other hand, patients older than 60 years who received medical management alone showed better survival probability than those who underwent surgery, however, the survival curves did not show statistical significance [Table/Fig-5]. Thus, in Indian settings, we did not observe a clinical benefit with DHC in mMCAI among older people.
Comparison of mortality rate among patients with age ≤60 and >60 years.
| No. of patients | No. of deaths | Death rate | p-value | OR(CI) |
|---|
| At three months |
| Age ≤ 60 years:MedicalSurgical | 2214 | 152 | 68%14% | 0.004 | 12.85(2.244-73.635) |
| Age > 60 years:MedicalSurgical | 186 | 63 | 33%50% | 0.469 | 0.5(0.076-3.26) |
| At six months |
| Age ≤ 60 years:MedicalSurgical | 2214 | 152 | 68%14% | 0.004 | 12.85(2.244-73.635) |
| Age > 60 years:MedicalSurgical | 186 | 74 | 39%67% | 0.248 | 0.318(0.045-2.223) |
OR: Odds ratio; CI: Confidence intervals
Comparison of survival probability among patients with age ≤60 and >60 years.
Disability Analysis
Good functional outcome as defined by mRS≤3 at six months was more frequent in DHC group (6/20, 30%) as compared with medical management group (10/40, 25%), yet the difference was not statistically significant. At three months however, mRS≤3 was equally observed in both the groups [Table/Fig-6]. Thus, functional outcome in DHC group improved with time, although in a non-significant manner. Most of the patients undergoing surgery lived with moderately severe disability (mRS=4) which was observed in 35% (vs 18% in medical group, p=0.15) of the patients at six months.
Proportional functional outcome analysis among survivors.
| Medical management (n=40) | Surgical management (n=20) | Significance (p-value) |
|---|
| At three months | | | |
| mRS≤3(%) | 6 (31.6%) | 3 (20%) | 0.483 |
| mRS=4(%) | 11 (57.9%) | 10 (66.7%) | 0.625 |
| mRS=5(%) | 2 (10.5%) | 2 (13.3%) | 0.818 |
| At six months | | | |
| mRS≤3(%) | 10 (55.5%) | 6 (42.9%) | 0.503 |
| mRS=4(%) | 7 (38.9%) | 7 (50%) | 0.555 |
| mRS=5(%) | 1 (5.6%) | 1 (7.1%) | 0.875 |
mRS: modified Rankin score
Continuous variables were compared using ‘Student’s t-test’ and categorical variables were compared using ‘Chi-square test’ for descriptive analysis
Proportional Functional Outcome Analysis among Survivors
Among the survivors, good functional outcome (mRS≤3) was observed in 55.5% of the patients on best medical management alone in comparison to 42.9% of the DHC group patients at six months follow up [Table/Fig-6]. Thus, higher number of patients who survived after the medical management alone achieved a good functional outcome in comparison to the survivors of the surgery group. This difference was though, not statistically significant (p=0.503). Further, at the end of follow up period, 50% of the patients in DHC group lived with moderately severe disability (mRS=4) in comparison to 38.9% of the patients who received best medical management alone. The difference again, was not statistically significant (p=0.555). Only one patient in each group lived with a severe disability (mRS=5) at six months [Table/Fig-6]. Thus, severe disability among the survivors of either medical or surgical management groups was infrequent.
We did not perform a separate subgroup analysis for functional outcome among patients with age ≤60 and >60 years because only two patients in the DHC group with age more than 60 years survived. Hence, survivors of the DHC group were mainly a representation of the patients with age ≤ 60 years.
Independent Predictors of Survival
Cox regression technique was used to analyse adjusted survival analysis in the combined study population. ‘Medical management alone’ and ‘midline shift>5 mm’ were observed as independent predictors of poor survival (p=0.007 and p<0.001 respectively).
Discussion
This study reiterates the previous data that DHC in mMCAI improves the outcome in terms of mortality risk. Nevertheless, the death rate reduction after DHC at the end of six months failed to reach the significance levels, unlike the previous studies. The probable cause for this non-significant mortality benefit appeared to be a differential outcome among the patients of age ≤60 and >60 years. The subgroup analysis of the two age groups revealed a significant reduction in death rate with surgery among the former (14% vs 68%, p=0.004) while an increased mortality rate among the later, in comparison with the best medical management alone. So, elderly patients having a higher death rate in the DHC group probably had a counteracting effect on the mortality benefit achieved in younger patients, thus bringing down the entire significance level. The higher mortality rate in patients older than 60 years among DHC group is in remarkable contrast with the results of two western world randomised clinical trials (DESTINY II and HeADDFIRST) which reported significant reduction in deaths after surgery [12,13]. It was on the basis of above two randomized trials that the 2015 update of Korean clinical practice guideline for decompressive craniectomy in mMCAI was released with inclusion of recommendation of surgery in patients older than 60 years (level of evidence Ia, grade of recommendation A) [10]. Rai V et al., from India reported a significant reduction in death rate after DHC in mMCAI, however, their subject population allocated to surgery group composed mostly of patients less than 60 years (mean age: 44.6±12 years) [6]. Hence, this is the first research from India to have studied the effects of DHC in mMCA infarction in elderly (>60 years) as well as younger (≤60) patients. This study shows higher death risk after surgery in elderly, which may suggest that the mortality benefit as achieved in the western clinical trials and the consequently concerning update in Korean DHC guidelines may not hold its value in Indian health settings. This may be the case with other developing countries as well where the level of post-operative care and infection control may not match with western standards. Thus, whether patients older than 60 years with mMCAI should be operated or not-remains to be answered and a well-designed randomised clinical trial in Indian setting, is needed to provide a convincing answer for the same.
Good functional outcome (mRS≤3) at six months was more commonly observed in the surgery group (30% vs 25%) but the difference was not statistically significant. Earlier, DESTINY trial had shown good outcome (mRS≤3) with DHC in mMCAI but the results could not reach statistical significance (p=0.23) [14]. Though, the meta-analyses of major RCT’s in western world have shown significant increase in patients achieving good outcome (mRS≤3) after surgery, the results of the present study could not reach statistical significance probably because of lesser sample size of DHC group patients [8,9].
Further, we believed that the realistic analysis of functional outcome would be to assess it among survivors. So, we performed a proportional analysis of the same among the surviving cohort. The patients undergoing best medical management alone showed a higher proportion of survivors having good functional outcome (mRS≤3) at six months, in comparison to those undergoing DHC (55% vs 43%), however the difference was not significant. Besides, higher proportion of the patients undergoing surgery (50% vs 39%) lived with moderately severe disability (mRS=4) while only one patient in both the groups lived with severe disability (mRS=5). However, among the surgical group, these functional outcome results are mainly a reflection of patients ≤60 years of age as only two patients with age >60 years survived. Since mRS score of 4 implies that a patient can’t walk or attend to his own bodily needs without assistance, we didn’t actually consider it as a measure of good functional outcome as has been previously debated by some authors [7]. So, it can be impressed from the results of the present study cohort that the best medical management alone in young patients with mMCAI either results in death or survival with somewhat (non-significant) better probability of good functional outcome. On the other hand, surgery offers a significantly higher chance of survival but with a non-significant increase in the probability of moderately severe disability among survivors. Howbeit, patients with age more than 60 years in our population do not appear to have any benefit with surgery, per contra, may have higher chances of death after surgery. The recent data from the western world though shows improved survival rate with surgery in patients more than 60 years of age; however, reported that the majority of survivors were left with major disability (mRS4-5) [15]. Functional outcomes after surgery as observed in young population in the present study are in line with the results of three previously published meta-analyses which showed a non-significant increase in proportion of survivors with major disability after DHC in mMCAI [8,9,16]. Our results matched closely with the observations of a meta-analysis by Vahedi K et al., which pooled the results of three prominent randomized clinical trials (DECIMAL, DESTINY AND HAMLET) and showed that DHC in mMCAI increases the probability of living a physically dependent life, although the risk of severe disability (mRS=5) does not increase with surgery [9]. Nevertheless, the results of this study regarding functional outcomes in survivors are different from the previous Indian study, which showed that none of the survivors in the medical management group had good outcome (mRS≤3) [6]. A large scale randomized clinical trial in India appears to be warranted to test if the results observed in the western world are reproducible in Indian clinical settings.
Limitation
Limitations of our study include a smaller sample size of the surgery group and a shorter follow up period. The results of the present study need to be compared with further studies in India or other developing countries, having larger number of mMCAI patients undergoing DHC and with a longer follow up (12 months) period. Further, our study had only six patients older than 60 years in the surgery group and the observation of increased mortality rate in elderly after surgery needs to be tested by enrolling larger number of such patients for surgery.
Conclusion
DHC in mMCAI patients in our study re-confirmed the established western data of significant reduction in death rate after surgery albeit only in patient’s ≤60 years of age. On the other hand, patients older than 60 years had a higher mortality rate after surgery. Good functional outcome was more frequently observed among the survivors of medical management group, though the difference was not statistically significant. Most of the DHC group survivors lived with moderately severe disability.