Mycobacterium tuberculosis is a common infection in developing countries with high morbidity and mortality. However, Non-Tuberculous Mycobacteria (NTM) are common environmental organisms which closely resemble tuberculosis but rarely present with disease in immunocompromised patients. They have different growth requirements, identification methods and treatment options when compared to tuberculosis and require individualised care. Here we present a case of Mycobacterium simiae infection in an immunocompetent patient presenting like tubercular infection with good outcomes after treatment.
Introduction
Non-tuberculous mycobacteria are widely distributed in the environment with high isolation rates worldwide [1,2]. Organisms can be found in soil and water, including both natural and treated water sources. Prevalence of NTM was highest among Japanese, Chinese, and Vietnamese patients (>300/100,000 persons) and lowest among Native Hawaiians and other Pacific Islanders (50/100,000) [3]. Currently, there are more than 150 species of NTM identified worldwide. By far, the most common organism associated with pulmonary disease is Mycobacterium avium Complex (MAC). Others are Mycobacterium kansasii, M. abscessus [4,5]. However, Mycobacterium simiae is a rare cause of pulmonary infections. Here, we present a case of M.simiae causing pulmonary infection in immune competent host.
Case Report
A 45-year-old female with no co-morbid conditions presented to a local private hospital in November 2015 with complaints of cough with minimal mucoid expectoration of one month duration and minimal haemoptysis for 5 days duration. Patient underwent bronchial artery embolisation and subsequently was found to be sputum positive for acid fast bacilli and GeneXpert® did not detect MTB complex. Hence, possibility of NTM was considered and she was started on isoniazid, rifampicin, and clarithromycin. However, patient did not improve symptomatically even after 10 months of above regimen.
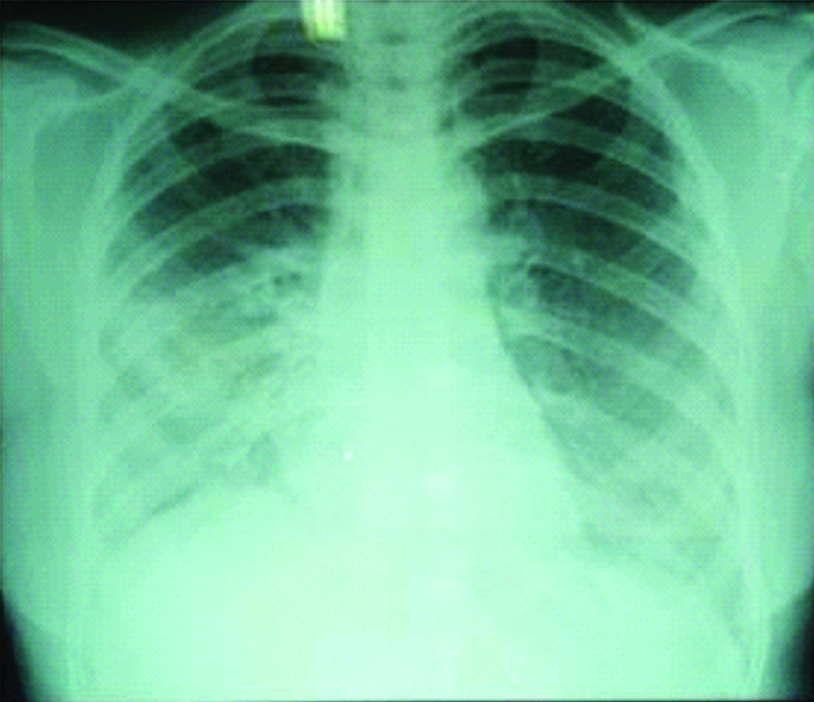
Then she came to our hospital in the month of September 2016 with complaints of cough with minimal mucoid sputum, low grade fever, loss of appetite and loss of weight. We ordered a chest radiograph which showed non-homogeneous opacity in right lower zone [Table/Fig-1]. We advised further evaluation but patient did not come for review.
Chest radiograph showing non homogeneous opacity in right lower zone.
Patient again came to our hospital in the month of June 2017 with the same complaints. On examination, patient was oriented to time, place and person. No pallor, clubbing, cyanosis, lymphadenopathy, icterus and pedal oedema were observed. Her vitals were pulse rate 98/minute, respiratory rate 23/minute, blood pressure 110/70 mmHg, SpO2 98% at room air. Auscultation of chest revealed bilateral normal vesicular breath sounds. Cardiovascular, abdominal and nervous system examination was normal. Complete blood picture showed haemoglobin of 12 gm%, total leukocyte count 7600 cells per cumm with normal differential count. Renal and liver function tests were normal. Viral screening was normal. Echocardiogram was normal. High resolution computed tomography showing bronchiectasis in right upper lobe and thick walled cavitory lesion in apical segment of right lower lobe [Table/Fig-2]. Sputum analysis did not reveal acid fast bacilli. Mantoux test was <5 mm. Bronchial washes were smear positive for acid fast bacilli and Gene Xpert® did not detected MTB complex. Based on above findings, possibility of non-tuberculous mycobacteria was considered and specimen sent for BACTEC® culture.
High resolution computed tomography showing bronchiectasis in right upper lobe and thick walled cavitory lesion in apical segment of right lower lobe.

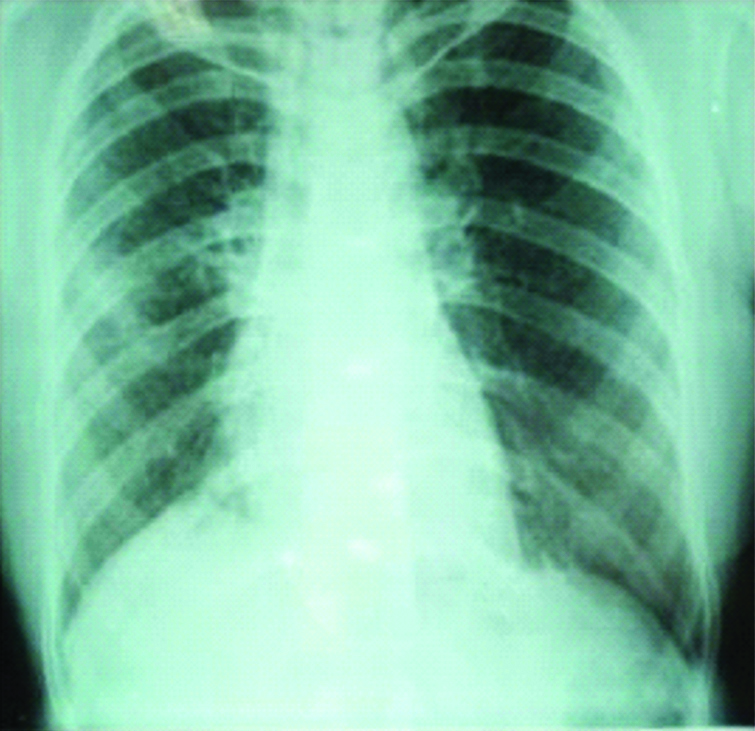
After four weeks, culture showed growth of NTM and species was identified as Mycobacterium simiae by DNA sequencing. After searching literature, Moxifloxacin and Trimethoprimsulphomithoxazole were added to above regimen. After six months the patient improved symptomatically with good radiological resolution [Table/Fig-3] and as she was not producing sputum further smear and culture examination could not be carried out.
Chest radiograph showing patient improvement after six months.
Discussion
Mycobacteria are a family of small, rod-shaped bacilli that can be classified into two main groups: 1) Mycobacterium tuberculosis complex: consisting of tuberculosis, leprae, bovis, africanum; 2) NTM.
Although over 150 different species of NTM have been described, pulmonary infections are commonly due to MAC, Mycobacterium kansasii, and Mycobacterium abscessus.
In 1959, botanist Ernest Runyon put these human disease-associated bacteria into four groups Photochromogens, Scotochromogens, Non-chromogens, and Rapid growers [6]. M. simiae belongs to photochromogens which produce a yellow-orange pigment when exposed to light.
M. simiae was initially isolated from macacus rhesus monkeys in 1965 [7]. It was first reported in Israel and later cases were reported from Cuba, Southwestern America and Middle East. Narang P et al., reported three cases of M. simiae from blood of AIDS patients in 2005 [8]. M. simiae like other NTM is a ubiquitous organism with huge environmental reservoirs, such as natural and municipal water, soil, aerosols, protozoan, animals, and humans [9].
Like other NTM, M. simiae can cause pulmonary diseases, lymphadenitis, skin, soft tissue diseases, and disseminated diseases especially in AIDS patients. Maher M et al., reported a case of disseminated M. simiae infection in an immunocompetent patient. Pulmonary symptoms due to M. simiae are nonspecific. Infected patients frequently present with cough, sputum production, haemoptysis, sweating, weight loss, low grade fever, and dyspnoea. Patients with underlying lung diseases such as prior pulmonary tuberculosis or silicosis, chronic obstructive pulmonary disease, and non-cystic fibrosis bronchiectasis have higher risk for M. simiae than healthy people [10]. Our patient is immunocompetent and had no underlying chronic lung disease, presented with cough, sputum production, low grade fever, loss of weight and loss of appetite and she had haemoptysis previously.
Diagnosis is based on clinical and radiological findings consistent with pulmonary diseases and recurrent isolation of mycobacteria from sputum or isolated from at least one bronchial wash in symptomatic patients [4]. M. simiae can be cultured on Löwenstein-Jensen or Middlebrook media and typically requires four to six weeks to grow. Mycolic acid profile via HPLC enables discrimination between M. simiae, M. tuberculosis, and MAC [11]. Differentiation and characterisation of different NTM species is important for deciding the correct treatment regimen as advised by BTS guidelines [12]. Molecular testing, in conjunction with the key phenotypic test can be used for rapid and accurate identification of clinically relevant NTM species. PCR-restriction fragment length Polymorphism Analysis (PRA) of hsp65 and 16S rRNA sequencing are commonly used molecular tests for identification of NTM species [13].
Mycobacterium simiae is the most drug-resistant of all the NTM, and this may explain the very low cure rates associated with medical treatment alone [4]. M. simiae isolates are susceptible to fluoroquinolones and amikacin but resistant to all others including isoniazid, capreomycin, minocycline, doxycycline, p-aminosalicylic acid, and ethionamide [14]. However, some isolates are susceptible in vitro to trimethoprim-sulfamethoxazole and linezolid. There is no standard treatment for M. simiae, but the regimen containing clarithromycin, quinolones, ethambutol, and cycloserine seem to be effective. Regimen including Moxifloxacin, clarithromycin and a third drug to which the isolate is susceptible (like trimethoprime-sulfamtahoxazole, clofazimine, streptomycin, amikacin) is the best treatment approach [15]. Our patient received clarithromycin, moxifloxacin, trimethoprim-sulfamethoxazole, ethambutol and isoniazid with good clinical and radiological improvement.
Conclusion
M. simiae is a rare NTM species that can cause pulmonary infection. Commonly seen in immunocompromised patients but can also occur in immunocompetent individuals. Diagnosis is itself challenging in view of its close resemblance to tuberculosis. Definite species identification is important for appropriate treatment and prolonged antimicrobial treatment required based on drug susceptibility testing. However, prognosis is good provided specific therapy is offered.
[1]. Falkinham Jo, Nontuberculous mycobacteria in the environmentClin Chest Med 2002 23:520-51.10.1016/S0272-5231(02)00014-X [Google Scholar] [CrossRef]
[2]. Von Reyn CF, Waddell RD, Eaton T, Arbeit RD, Maslow JN, Barber TW, Isolation of Mycobacterium avium complex from water in the United States, Finland, Zaire, and KenyaJ Clin Microbiol 1993 1:3227-30. [Google Scholar]
[3]. Adjemian J, Frankland TB, Daida YG, Honda JR, Olivier KN, Zelazny A, Epidemiology of nontuberculous mycobacterial lung disease and tuberculosis, Hawaii, USAEmerg Infect Dis 2017 23(3):439-47.10.3201/eid2303.16182728221128 [Google Scholar] [CrossRef] [PubMed]
[4]. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseasesAm J Respir Crit Care Med 2007 175(4):367-416.10.1164/rccm.200604-571ST17277290 [Google Scholar] [CrossRef] [PubMed]
[5]. Yates MD, Grange JM, Collins CH, The nature of mycobacterial disease in south east England, 1977-84J Epidemiol Community Health 1986 40:295-300.10.1136/jech.40.4.2953655621 [Google Scholar] [CrossRef] [PubMed]
[6]. Runyon EH, Anonymous mycobacteria in pulmonary diseaseThe Medical clinics of North America 1959 43(1):273-90.10.1016/S0025-7125(16)34193-1 [Google Scholar] [CrossRef]
[7]. Baghaei P, Tabarsi P, Farnia P, Marjani M, Sheikholeslami FM, Chitsaz M, Pulmonary disease caused by Mycobacterium simiae in Iran’s national referral center for tuberculosisJ Infect Dev Ctries 2012 6:23-28.10.3855/jidc.129722240424 [Google Scholar] [CrossRef] [PubMed]
[8]. Narang P, Narang R, Mendiratta DK, Roy D, Deotale V, Yakrus MA, Isolation of Mycobacterium avium complex and M. simiae from Blood of aids patients from sevagram, MaharashtraIndian J Tuberc 2005 52:21-26. [Google Scholar]
[9]. Makovcova J, Slany M, Babak V, Slana I, Kralik P, The water environment as a source of potentially pathogenic mycobacteriaJ Water Health 2014 12:254-63.10.2166/wh.2013.10224937219 [Google Scholar] [CrossRef] [PubMed]
[10]. Balkis MM, Kattar MM, Araj GF, Kanj SS, Fatal disseminated Mycobacterium simiae infection in a non-HIV patientInt J Infect Dis 2009 13:e286-87.10.1016/j.ijid.2008.10.01519155183 [Google Scholar] [CrossRef] [PubMed]
[11]. Leite CQ, da Silva Rocha A, de Andrade Leite SR, Ferreira RM, Suffys PN, de Souza Fonseca L, A comparison of mycolic acid analysis for nontuberculous mycobacteria identification by thin-layer chromatography and molecular methodsMicrobiol Immunol 2005 49(7):571-78.10.1111/j.1348-0421.2005.tb03642.x16034199 [Google Scholar] [CrossRef] [PubMed]
[12]. Haworth CS, Floto RA, Introducing the new BTS Guideline: Management of non-tuberculous mycobacterial pulmonary disease (NTM-PD)Thorax 2017 72(11):969-70.10.1136/thoraxjnl-2017-21092929054887 [Google Scholar] [CrossRef] [PubMed]
[13]. Shojaei H, Heidarieh P, Hashemi A, Feizabadi MM, Daei Naser A, Species identification of neglected nontuberculous mycobacteria in a developing countryJpn J Infect Dis 2011 64:265-71. [Google Scholar]
[14]. Li G, Lian LL, Wan L, Zhang J, Zhao X, Jiang Y, Antimicrobial susceptibility of standard strains of nontuberculous mycobacteria by micro plate Alamar Blue assayPLoS One 2013 8:e8406510.1371/journal.pone.008406524386332 [Google Scholar] [CrossRef] [PubMed]
[15]. Maoz C, Shitrit D, Samra Z, Peled N, Kaufman L, Kramer MR, Pulmonary Mycobacterium simiae infection: comparison with pulmonary tuberculosisEur J Clin Microbiol Infect Dis 2008 27:945-50.10.1007/s10096-008-0522-618488259 [Google Scholar] [CrossRef] [PubMed]