A Rare Case of Poorly-Differentiated Sertoli Leydig Cell Tumour of Ovary with Mesenchymal Heterology
Rahul Pandey1, Yasmeen Khatib2, Vinita Pandey3, Archana Khade4, Manisha Khare5
1 Ex-Registrar, Department of Pathology, TATA Memorial Hospital, Parel, Mumbai, Maharashtra, India.
2 Associate Professor, Department of Pathology, HBT Medical College and Dr. R.N. Cooper Hospital, Juhu, Mumbai, Maharashtra, India.
3 Ex Speciality Medical Officer, Department of Pathology, HBT Medical College and Dr. R.N. Cooper Hospital, Juhu, Mumbai, Maharashtra, India.
4 Assistant Professor, Department of Pathology, HBT Medical College and Dr. R.N. Cooper Hospital, Juhu, Mumbai, Maharashtra, India.
5 Professor, Department of Pathology, HBT Medical College and Dr. R.N. Cooper Hospital, Juhu, Mumbai, Maharashtra, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Archana Khade, Department of Pathology, 1nd Floor, ‘C’ Wing, HBT Medical College and Dr.R.N. Cooper Hospital, Juhu, Mumbai-56, Maharashtra, India.
E-mail: arck115@gmail.com
Sertoli–Leydig Cell Tumours (SLCT) accounts for less than 0.5% of all ovarian neoplasms. Presence of mesenchymal heterologous elements in a poorly differentiated SLCT is extremely uncommon. It not only causes diagnostic difficulty but also renders an aggressive behaviour to the tumour. We report a rare case of poorly differentiated SLCT with cartilage and rhabdomyoblastic differentiation along with review of literature.
Cartilage, Heterologous elements, Ovarian tumour, Rhabdomyoblastic differentiation
Case Report
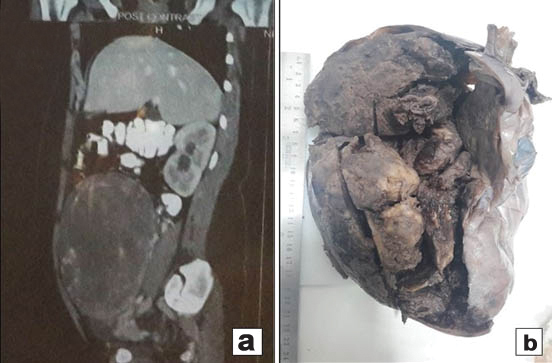
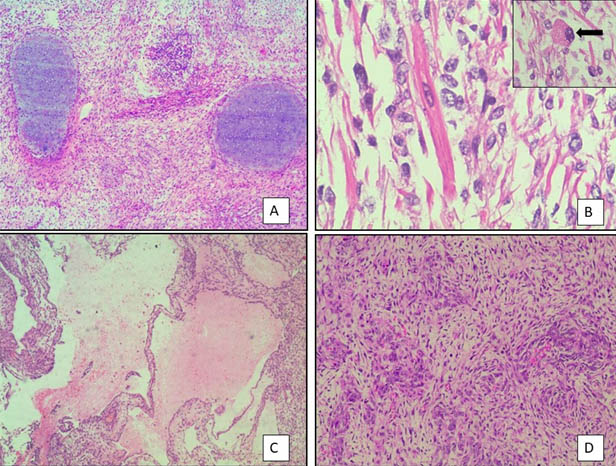
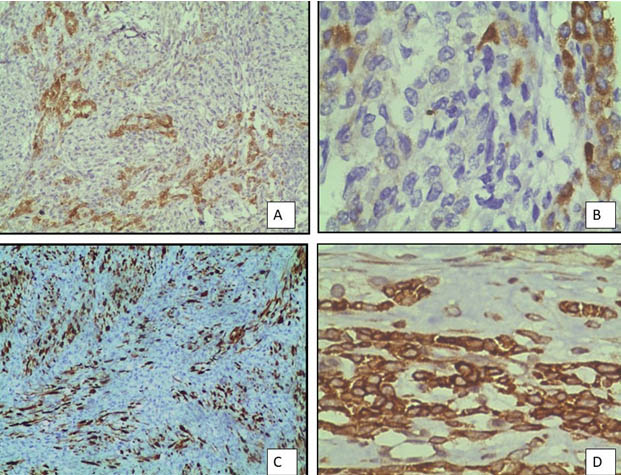
A 17-year-old unmarried woman presented with secondary amenorrhoea of two months duration along with lower abdominal pain and distension. Abdominal examination revealed a mass of 20 weeks gestation size. Computed tomography showed a 16×10 cm solid cystic mass with multiple septae in the right adnexal region [Table/Fig-1a]. Her CA 125 levels were elevated at190 u/l and Serum beta-HCG and AFP levels were normal. Patient underwent right salpingo-ophorectomy. Grossly, tumour measured 18x10x6.5cm and had a smooth and congested capsule. Cut-section was solid and cystic with variegated appearance with grayish white areas along with haemorrhage, necrosis and translucent areas of cartilage [Table/Fig-1b]. Microscopy showed a tumour predominantly composed of spindle cells with foetal type cartilage [Table/Fig-2a]. At places foci of skeletal muscle differentiation in the form of strap cells and cells resembling rhabdomyoblasts were discernable [Table/Fig-2b]. The mitotic count in the most cellular area was approximately 2-3/10 High Power Fields (HPF). There was presence of anastamosing channels resembling vascular spaces along with retiform areas [Table/Fig-2c]. A minor component of small oval cells with scant cytoplasm and hyperchromatic nuclei arranged in nests cords and tubules were seen [Table/Fig-2d]. These tumour cells displayed brisk mitosis of 2-3/10 hpf. In view of the histomorphology, a diagnosis of teratoma was initially considered. However, on Immunohistochemistry (IHC), tumour cells expressed calretinin, inhibin alpha and pan cytokeratin [Table/Fig-3a-c]. They were negative for epithelial membrane antigen and synaptophysin. The stains also highlighted the presence of scanty, peripherally located leydig cells which were missed on haematoxylin and eosin stain. The spindle cell areas expressed desmin and focal myogenin [Table/Fig-3d]. A final diagnosis of poorly differentiated SLCT with heterologous cartilage and rhabdomyoblastic differentiation was given based on morphology and immunohistochemistry. The patient was advised adjuvant chemotherapy and close follow-up. On follow-up the patient was free of disease, one year post-treatment.
a) CT Scan- showing a large solid cystic adnexal mass with multiple septae; b) Gross appearance - showing solid cystic tumour with variegated appearance and haemorrhage.
Histopathogical features: a) Low power view showing tumour with spindle cells and foetal type of cartilage. (H&E-10x); b) High power view showing strap cells with rhabdomyoblast (inset). (H&E-40x); c) Low power view showing tumour with retiform areas. (H&E-10x); d) High power view showing immature sertoli cells in sheets and nests with small dark nuclei.
Immunohistochemistry profile of tumour: a) calretinin positivity (IHC-10X); b) Focal inhibin positivity. (IHC-40X); c) Pan cytokeratin positivity (IHC-10X); d) spindle cells showing desmin positivity (IHC-40X).
Discussion
The presence of heterologous elements in poorly-differentiated SLCT can be of endodermal or mesenchymal types [1-3]. The earliest case of heterologous elements in SLCT was reported by Mayer in 1930, who described the presence of mucinous epithelium [2]. Endodermal heterology with presence of GIT epithelium, carcinoid-like areas and cells resembling hepatocytes have been more commonly described [3]. However, on review of literature till now only 23 cases of PDSLCT with mesenchymal heterology have been reported [Table/Fig-4] [2,4-11]. In 1982, Prat J et al., reviewed 190 cases of SLCT and found only 12 cases with mesenchymal heterology with presence of immature cartilage, skeletal muscle and neuroblastoma in one case [2]. Though age group of reported cases ranged from 11 -70 years, most patients were young females which was similar to our case. The commonest clinical presentation was the presence of rapidly growing abdominal mass. A 7/23 patients presented with features of virilization [2,5] and 9/23 patients presented with amenorrhoea [2,7,8,11]. Our patient presented with rapidly increasing abdominal mass and secondary amenorrhoea. The occurrence of mesenchymal components is often associated with extraovarian spread, recurrence and aggressive behaviour. In our literature review, 14/23 patients presented with recurrence [2,5,9,10] and 10/23 died [2] inspite of adjuvant chemotherapy. This finding is in contrast to pure SLCT and those with endodermal elements where majority of the tumours are stage IA with favourable prognosis [1,3]. On gross examination these tumours tend to be more cystic than solid in comparison to well-differentiated SLCT [1-3]. On microscopy they display a wide range of histological spectrum with mixture of spindle cells, skeletal muscle, rhabdomyosarcomatous component, cartilage and are prone to misinterpretation due to presence of admixture of components. The areas of sertoli cell differentiation are composed of small clusters and cords of dark blue cells along with few leydig cells. The differential diagnosis of SLCT with mesenchymal heterologous elements includes teratoma, primary ovarian sarcomas (rhabdomyosarcoma, chondrosarcoma) and Malignant Mixed Mullerian Tumour (MMTA). Teratoma contains a wide range of elements including skin and its appendages, neuroepithelium and glial tissue and lacks presence of sertoli leydig cells. Primary ovarian sarcomas are present in older females and tend to be solid. Further, areas of skeletal muscle differentiation are less pleomorphic, more mature with a lesser mitotic count as compared to PDSCLT [2]. Metaplastic cartilage in SLCT is also less cellular and resembles foetal-type cartilage [2]. Malignant Mixed Mullerian Tumour (MMTA) is ruled out due to absence of carcinomatous component and Epithelial Membrane Antigen staining (EMA) [2]. Patients of PDSCLT are young and present with a progressive abdominal enlargement with symptoms of virilization which gives a clue about its origin. Microscopically our case was initially diagnosed as a teratoma due to the presence of cartilage and retiform areas which were mistaken for vascular channels. However, focal areas showed sertoli leydig cells which were confirmed on IHC. IHC is thus an indispensible diagnostic tool in these cases. Positive staining with Inhibin alpha, calretinin, cytokeratin and EMA negativity helps in confirming the diagnosis. Optimal treatment for these tumours is hysterectomy with bilateral salpingo oophorectomy. However, due to rarity of the tumour; there are no standardised protocols for adjuvant chemotherapy [10]. Prat J et al., stated that the prognosis of SLCTs with mesenchymal elements is as poor as primary ovarian sarcomas and hence adjuvant chemotherapy is imperative for all disease stages [2]. Our literature review also highlights the aggressive behaviour of these tumours. Hence, our patient was advised adjuvant chemotherapy after right ovarian cystectomy.
Reported cases of poorly differentiated ovarian sertoli-leydig cell tumours with mesenchymal heterology.
| Author | Age | Symptoms | Type of heterologus elements | Treatment | Recurrence if any | Recurrance after time interval | Treatment after recurrence | Follow-up |
|---|
| Kanter & klawans [2] | 33 | A,V,M | Cartilage, GI epi | USO | Nil | - | | Disease free at 5 months |
| Krock & Wolferman [2] | 18 | A,V | Cartilage | USO | Omentum and right ovary | 13m | | Died at 17 months |
| Hughesdon & Fraser [2] | 53 | V, M | Skeletal M, GI epi | TAH with BSO | Pelvis | 6m | | Died at 8 months |
| Prat J et al., [2] | 32 | A,M | skeletal M, cartilage | TAH with USO | Peritoneum | 5 m | - | Died after 5 months |
| Prat J et al., [2] | 11 | M | Cartilage, GI epi | USO | - | - | - | Alive after 10 years |
| Prat J et al., [2] | 22 | M | Skeletal M | Biopsy | - | - | - | Not known |
| Prat J et al., [2] | 17 | M | Skeletal M, GI epi | U SO | Right ovary and pelvis | 6m | - | Died after 12 months |
| Prat J et al., [2] | 20 | A,V,M | Skeletal M | TAH+BSO | peritoneum | 5m | SX+CT+RT | Died after 10 months |
| Prat J et al., [2] | 36 | M | Skeletal M, cartilage | USO | peritoneum | 5 m | - | Died after 6 months |
| Prat J et al., [2] | 16 | A | Skeletal M | USO | Left ovary and peritoneum | 6m5Yrs | SX+CT | Died after 7 years |
| Prat J et al., [2] | 20 | V,M | Skeletal M | USO+ CT | Left ovary | 4 m | SX+RT | Died after 18 months |
| Prat J et al., [2] | 14 | M | Cartilage | USO | Pelvis | 3m | RT | Died after 9 months |
| Prat J et al., [2] | 24 | M | Cartilage, GI epi | USO | Omentum and pelvis | 5 m | SX +CT | Died after 18 months |
| Prat J et al., [2] | 48 | A,M | Skeletal M, cartilage | TAH+USO | - | - | Not known | Not known |
| Prat J et al., [2] | 23 | V, M | Cartilage, GI epi, skeletal M | USO | Peritoneum and pelvis | 1 year | CT | Alive and disease free at 2 years |
| Talerman A et al., | 22 | - | Skeletal M | USO | - | | - | Disease free after 10 months |
| Grove A et al., [5] | 29 | M | Cartilage, RMS | USO | - | | - | Disease free after 4 yrs |
| Guerard MJ et al., [6] | 16 | V,M | RMS | USO | Abdomen | 6 m | SX+CT | |
| Brightwell R et al., [7] | 31 | A,M | RMS | USO+CT | - | - | - | Disease free at 18 months |
| Sahoo TK et al., [8] | 27 | A,M | Bone, GI epi | USO+CT | - | - | - | - |
| Papler T et al., [9] | 70 | M | RMS | TAH BSO | Widespread | 7m | - | - |
| Rekhi B et al., [10] | 17 | M | RMS, skeletal M | USO | Omentum | 1 yR | CT | |
| Choughle A et al., [11] | 23 | A, M | RMS hepatocytes | USO | - | - | - | - |
V = virlization A= amenrrhoea, M=abdominal mass, USO = Unilateral salpingo-ophorectomy, TAH with BSO= Total abdominal hysterectomy with bilateral sapingoophorectomy, CT = Chemotherapy, SX =surgery Skeletal M=Skeletal muscle, GI epi=Gastrointestinal epithelium, RMS=rhabdomyosarcoma
Conclusion
Sertoli Leydig cell tumours with mesenchymal heterology are very rare and should be included in the differential diagnosis of a cystic teratoma because of their morphological similarities but completely different therapeutic implications. Extensive tumour sampling is imperative as sertoli leydig cell component may be missed and these tumours have aggressive behaviour and recurrence potential.
V = virlization A= amenrrhoea, M=abdominal mass, USO = Unilateral salpingo-ophorectomy, TAH with BSO= Total abdominal hysterectomy with bilateral sapingoophorectomy, CT = Chemotherapy, SX =surgery Skeletal M=Skeletal muscle, GI epi=Gastrointestinal epithelium, RMS=rhabdomyosarcoma
[1]. Young RH, Scully RE, Ovarian Sertoli-Leydig cell tumours. A clinicopathological analysis of 207 casesAm J Surg Pathol 1985 9:543-69.10.1097/00000478-198508000-000013911780 [Google Scholar] [CrossRef] [PubMed]
[2]. Prat J, Young RH, Scully RE, Ovarian Sertoli-Leydig cell tumours with heterologous elements. II. Cartilage and skeletal muscle: A clinicopathologic analysis of twelve casesCancer 1982 50:2465-75.10.1002/1097-0142(19821201)50:11<2465::AID-CNCR2820501135>3.0.CO;2-U [Google Scholar] [CrossRef]
[3]. Young RH, Prat J, Scully RE, Ovarian Sertoli-Leydig cell tumours with heterologous elements. I. Gastrointestinal epithelium and carcinoid: A clinicopathologic analysis of thirty-six casesCancer 1982 50:2448-56.10.1002/1097-0142(19821201)50:11<2448::AID-CNCR2820501133>3.0.CO;2-T [Google Scholar] [CrossRef]
[4]. Kostopoulou E, Talermasn A, Ovarian Sertoli-Leydig cell tumour of intermediate differentiation with immature skeletal muscle heterologous elementsActa Obstet Gynecol Scand 2003 82:197-98.10.1034/j.1600-0412.2003.00021.x12648187 [Google Scholar] [CrossRef] [PubMed]
[5]. Grove A, Vestergaard V, Ovarian Sertoli-Leydig cell tumour of intermediate grade with heterologous elements of rhabdomyosarcoma. A case report and a review of the literatureAnn Diagn Pathol 2006 10:288-93.10.1016/j.anndiagpath.2005.09.01216979522 [Google Scholar] [CrossRef] [PubMed]
[6]. Guérard MJ, Ferenczy A, Arguelles MA, Ovarian Sertoli-Leydig cell tumour with rhabdomyosarcoma: An ultrastructural studyUltrastruct Pathol 1982 3:347-58.10.3109/019131282090185577157497 [Google Scholar] [CrossRef] [PubMed]
[7]. Brightwell R, Grzankowski K, Kasznica J, Frederick PJ, Poorly differentiated Sertoli–Leydig tumour with heterologous, high-grade, sarcomatoid features: A case reportGynecologic Oncology Reports 2015 14:6-8.10.1016/j.gore.2015.08.00126793762 [Google Scholar] [CrossRef] [PubMed]
[8]. Sahoo TK, Kar T, Kar A, Panda S, Poorly differentiated sertoli-leydig cell tumour of ovary with heterologous elementsJournal of Clinical and Diagnostic Research: JCDR 2017 :1110.7860/JCDR/2017/25262.986028658892 [Google Scholar] [CrossRef] [PubMed]
[9]. Burnik Papler T, Frković Grazio S, Kobal B, Sertoli - Leydig cell tumour with retiform areas and overgrowth of rhabdomyosarcomatous elements: case report and literature reviewJournal of Ovarian Research 2016 9:4610.1186/s13048-016-0257-427473538 [Google Scholar] [CrossRef] [PubMed]
[10]. Rekhi B, Karpate A, Deodhar KK, Chinoy RF, Metastatic rhabdomyosarcomatous elements, mimicking a primary sarcoma, in the omentum, from a poorly differentiated ovarian Sertoli-Leydig cell tumour in a young girl: An unusual presentation with a literature reviewIndian J Pathol Microbiol 2009 52(4):554-58.10.4103/0377-4929.5616519805972 [Google Scholar] [CrossRef] [PubMed]
[11]. Chougule A, Singh P, Kumar PS, Ovarian Sertoli–Leydig cell tumour with rhabdomyosarcoma and borderline mucinous neoplasmPathology 2016 48(3):78-281.10.1016/j.pathol.2016.02.01427020506 [Google Scholar] [CrossRef] [PubMed]