Peripheral vascular catheters are frequently accessed for administration of medications. The catheter is flushed with 0.5 to 1ml of normal saline as a continuous push over 30 to 60 seconds after each medication to ensure complete administration of medications as well as to provide a saline lock. This saline lock is intended to prevent hub contamination and occlusion of the catheter with blood. However, this practice is not full proof. Earlier study by the same authors had shown that the Peripheral Intravenous Catheter (PIV) hub was contaminated with the blood despite connecting needle-less venous access bungs such as Bionector [1]. Blood contaminated hub can act as a nidus for infection [2] and hence keeping the catheter hub clear of blood is important.
Several studies are conducted in adult population with central catheters to decrease contamination of catheter hub. Pulsatile flushing rather than continuous push has shown to reduce the catheter hub contamination [3-5]. Centre for Disease Control (CDC) recommends flushing the central venous catheters in pulses rather than continuous push [6]. Similar studies are not done on peripheral intravenous and small bore catheters used in neonatal intensive care units. This is compounded by the fact that the volume of flush used in neonatal intensive care units is lot less than that used in adult population with central catheters. This study aimed to compare the effectiveness of continuous flush technique with pulse flush technique in clearing the blood contaminated peripheral vascular catheter hub.
Materials and Methods
A single blind cross over randomised control study was conducted in a tertiary level neonatal unit, between the months of November 2016 - January 2017at JSS Hospital, Mysuru, Karnataka, India.
Subjects: Health Care Professionals (HCP) with an experience of working in paediatric wards including Neonatal Intensive Care Unit (NICU) for at least one year were included. HCPs who had at least one year of experience and have not been working in paediatric/neonatal ward for previous one year were excluded. Each participant did both continuous and pulse technique to flush the contaminated catheter as per the randomisation order. Each participant had about 10 minutes to practice both techniques of flushing before they participated in the study.
Catheter: Small bore (24 G, 1.5 inches) Peripheral intravascular catheter.
Control: Continuous flush: A 1 ml of normal saline was loaded into a 2-cc disposable syringe. The syringe is fastened to the hub of the vascular catheter and continuous pressure is applied over the piston to empty the saline over 60 seconds.
Intervention: Pulse flush: A 1 ml of normal saline was loaded into the syringe as above. After fastening the syringe to the hub of the catheter, saline was pushed as pulses of 0.2ml each over a period of 60 seconds.
Randomisation: Simple randomisation for the order of flushing technique was adopted for this study. Random numbers sequence was generated using Microsoft excel by a staff not involved in the experiment. The sequence was secured in an opaque concealed envelope. The participant opened the envelope before the experiment and conducted flushing accordingly.
Experiment: Volume of the 24 G vascular catheter is estimated by flushing an empty catheter with normal saline and measuring the volume of the flush. This was found to be 0.2ml.
Blood sample in EDTA vacutainer about to be discarded was obtained from the pathology lab. One of the authors filled a new catheter with the blood using a syringe. Thus, filled syringe was handed over to the participant for flushing. The participant now flushed the catheter with one ml of normal saline and the flush was discarded. The technique of flushing (continuous or pulse) was as per the random sequence. After a washout period of 30 minutes, the same experiment was repeated with the other technique using a new catheter. Thus, flushed catheter was handed over back to the author, who obtained the hub residue by flushing the catheter with 0.2 ml of normal saline (which equals the volume of the catheter) followed by 1 ml of air flush into a EDTA vacutainer. The vacutainer was coded to blind the pathologist with respect to the flushing technique used. The collected hub residue fluid was immediately transferred to the pathology lab. This was analysed by the same pathologist for the RBC count. RBC count was done using Neubauer chamber and standard manual technique and calculations were done like in the study done earlier [1].
Statistics
Sample size: In our previous study, we noted the mean RBC count in the hub residue was 100000 [1]. We planned this study of a continuous response variable from paired control and experimental subjects. In a previous study the response within each subject group was normally distributed with standard deviation 25000 [1]. If the true difference in the experimental and control means is 10000 (10%), we needed to study 68 subjects to be able to reject the null hypothesis that the population means of the experimental and control groups are equal with probability (power) 0.9. The Type I error probability associated with this test of the null hypothesis was 0.05. We could recruit 64 subjects.
Primary outcome in this study was to compare the median RBC count in the hub residue.
Statistical Analysis
Continuous variables were summarised as median and interquartile range. Categorical variables were represented as proportions. Comparison of medians was done using Mann-Whitney U test. Single sample t-test was used to calculate the significance of mean values of differences between two techniques by the same participant. A p-value of less than 0.05 was taken as significant.
Results
We recruited a total of 64 subjects. A total of 55 of them were nursing staff and rest were junior doctors working in the paediatric department. Thirty-six of the participants were initially randomised to continuous flush technique and rest for pulse flush technique. Only six (9%) of the hub residue after continuous flush and five (8%) after the pulse flush appeared clear without blood tinge.
On microscopic examination, all 100% of the samples obtained by both techniques showed RBCs. Minimum and Maximum RBC counts with continuous flush was 8000 and 656000/cumm respectively. Similarly, minimum and maximum RBC counts with pulse flush were 10240 and 928000/cumm respectively. Overall, 34 (53%) of the samples obtained after pulse flush showed lower RBC count compared to continuous flush samples.
The median (interquartile range) of RBC count from samples obtained with continuous technique was 140000 (48000-218000) and that of samples obtained from pulse flush technique was 148000 (70000-226000)/cumm. The difference was not statistically significant with a p-value of 0.412. [Table/Fig-1] represents the distribution of RBC counts in the hub residue obtained by two different techniques.
Distribution of RBC counts in the hub residue obtained by two different techniques.
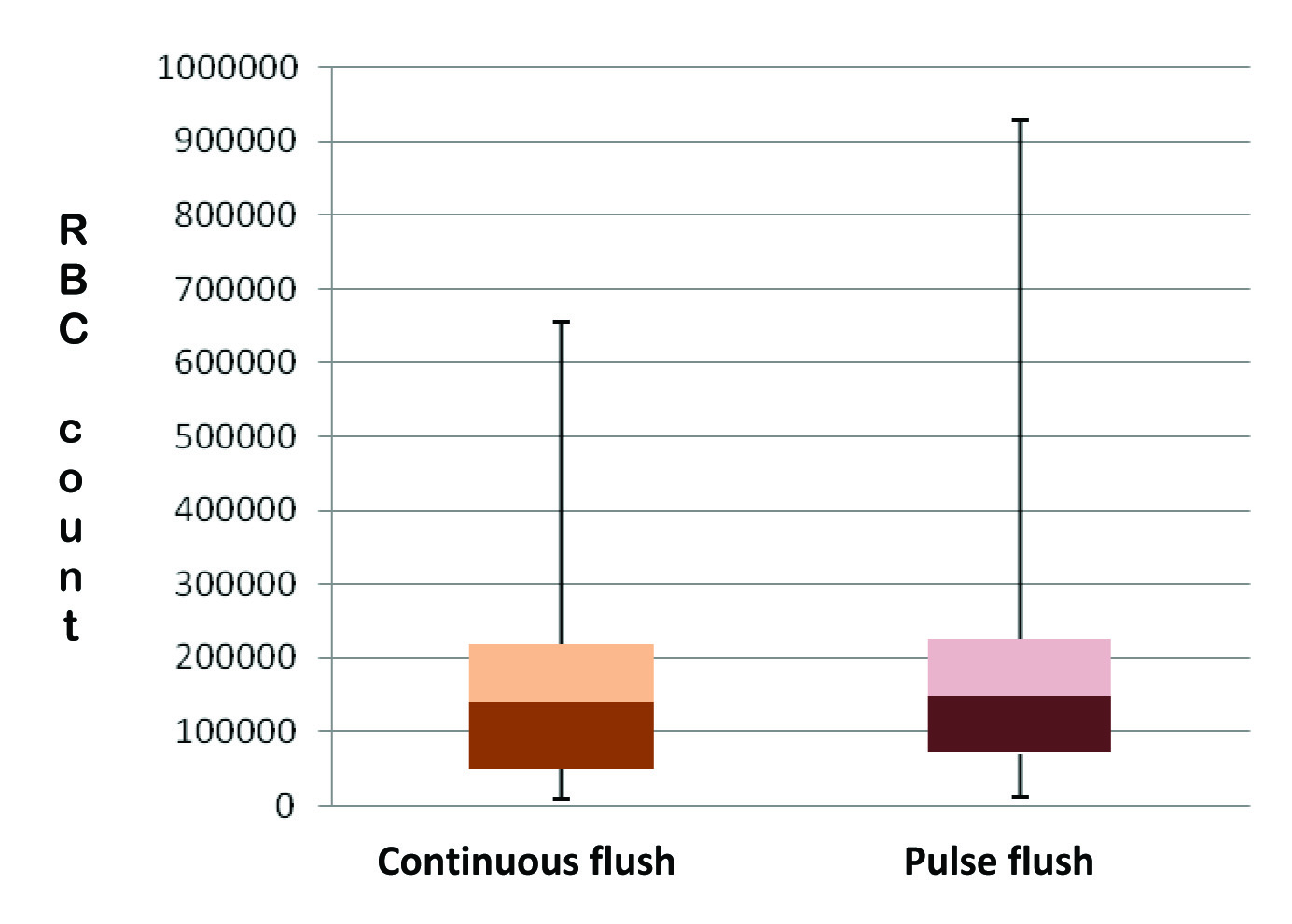
The mean of difference in RBC count between the continuous and pulse flush sample from the same participant was 22119/cumm with counts in the continuous flush being lower. The p-value was 0.399.
Discussion
PIV is the most common vascular access in neonatal intensive care unit. Our study shows there is no difference between the techniques used for flushing the catheter after use with respect to clearing the hub from blood.
Intravenous catheters are known source of nosocomial infection. Much of the studies are done on central venous catheters and hence, there is paucity of information on PIVs [7-9].
Many of the nursing practices in the neonatal unit are not uniform throughout out the world. This is particularly so regarding use of intravenous catheters [10,11]. Neonatal thrombophlebitis due to PIVs is a well-documented but under reported entity [7,12,13]. Whether variation in PIV catheter toilet has a role to play should be explored.
With studies in adult population and central venous catheter suggesting the benefit of pulse flushing [3,9], we examined if the same technique could be of benefit in small bore peripheral venous catheter in neonates. Results of our study do not suggest the superiority of pulse flushing over continuous flush when used in small bore PIVs and a small volume of flush. Clearing of the blood from the catheter hub is not only a function of pattern of flush fluid flow, but also of volume. The result of similar experiment could be different if a larger volume of flush fluid is used. Unfortunately, in neonatal intensive care, the elbow space available for using flush fluid liberally is almost non-existent due to stringent fluid requirement of sick babies.
This being an in vitro study, the results should be used with caution. Future in-vivo studies could be more informative in this regard.
Conclusion
The clearance of blood from the hub of a small bore PIV is not different in continuous and pulse flush.