Effect of Hydroalcoholic Extract of Arnebia Euchroma on the Treatment of Cutaneous Leishmaniasis
Roham Borazjani1, Shiva Aminnia2, Mohammad Rastegarian3, Mahnaz Hosseini4, Zahra Ghanbarinasab5, Soheil Ashkani-Esfahani6, Bahador Sarkari7
1 Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
2 Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
3 Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
4 Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
5 Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
6 Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran.
7 Department of Parasitology and Mycology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran; Basic Sciences in Infectious Diseases Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Bahador Sarkari, Department of Parasitology and Mycology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
E-mail: sarkarib@sums.ac.ir
Introduction
The growing resistance of the Leishmania parasites to the available anti-leishmanial drugs has been reported from different areas of the world, including Iran.
Aim
To assess the effect of hydroalcoholic extract of Arnebia euchroma, for treatment of cutaneous leishmaniasis induced by Leishmania major in BALB/c mice.
Materials and Methods
Arnebia euchroma extract was provided by medicinal herb garden in Yasuj, in the south of Iran. To facilitate the application of the extract, 5% gel with Arnebia extract and Carboxymethylcellulose (CMC) was prepared. Mice infection was done by subcutaneous inoculation of amastigotes form of Leishmania major. Lesions were developed after about three weeks of inoculating. Mice were divided into four groups, (six mice in each group), where the first group was treated with 5% of the extract and the second group with 5% gel of Arnebia euchroma. The third group was treated by meglumine antimoniate. The negative control group received only normal saline. Treatments was started from the day open lesion were observed and carried out every third day for 21 days. The size of lesion on tail base were measured weekly.
Results
Mean sizes of lesions in mice treated with the extract or topical gel were lower than the controls. However, the differences were not statistically significant (p>0.05).
Conclusion
Findings of the study showed that Arnebia euchroma extract in doses which have been used in this study (5%) and in comparison with meglumine antimoniate had no considerable effect in treatment or control of cutaneous leishmaniasis.
Carboxymethylcellulose (CMC), Meglumine antimoniate, Subcutaneous inoculation
Introduction
Cutaneous Leishmaniasis (CL) is a vector-borne and usually a self-limiting disease caused by the protozoan parasites called Leishmania. This parasite has two distinct structural variants: Amastigotes forms that are non-motile and intra-phagocytic form and Promastigotes that found in the alimentary tract of phlebotomine sand-flies. Worldwide, leishmaniasis has three major clinical manifestations. Among three main forms of leishmaniasis which are cutaneous, mucocutaneous and visceral forms, both Cutaneous Leishmaniasis (CL) and Visceral Leishmaniasis (VL) are endemic in central and south of Iran [1-6]. Fars, Ilam and Khorasan Razavi are among the most important endemic foci with a high prevalence of CL in Iran [1,2,5,7-9]. As an instance, 23% of annually reported cases of CL in Iran in 2009 were reported from Fars, according to the report of Iranian Ministry of Health. CL in Iran is mainly caused by L. major following by L. tropica [1,10]. Reduction in the duration of the healing process with minimal scar formation is the final goal of CL treatment. Glucantime and pentostam are still used as the drugs of choice for CL treatment but they exhibit a wide range of side effects such as nephrotoxicity, cardiotoxicity, and hepatotoxicity [11-13]. Hence, investigations for finding new drugs or methods that accelerate wound healing and reduce the scar formation in CL are in urgent needs.
Arnebia euchroma is one of the species of genus Arnebia related to the family Boraginaceae. Arnebia euchroma is an aboriginal herb in the Southeast of Iran and can be found in Himalaya too. It has been used for the treatment of burn wounds, skin ulcers in Chinese traditional medicine. Naphthoquinones are very remarkable chemical compounds found in the roots of Arnebia genus. Their fraction is made up of agents that are water-insoluble such as Shikonin and alkannin [14]. These compounds exhibit broad pharmacological properties including anti-inflammatory [15] and anti-viral effects [16]. The current study aimed to assess the effects of hydroalcoholic extract of Arnebia euchroma, for treatment of L. major induced cutaneous leishmaniasis in BALB/c mice.
Materials and Methods
Preparation of Arnebia Euchroma Gel
Arnebia euchroma extract was provided by Zardband Company of Yasuj, from medicinal herb garden in Yasuj, in the south of Iran. To facilitate the application of the agent, 5% gel with Arnebia extract, a concentration which was assigned according to a pilot study in our institute, was prepared by dissolving 5 g of Arnebia extract in 2 mL of distilled water. The solution was then transferred into 2% CMC (2 g CMC/ 100 mL of distilled water) for topical use. The CMC gel itself was administered on the CL lesion in a pilot study and had no significant effect on the lesion, comparing to the non-treated group, according to stereological parameters.
Inoculation of BALB/C Mice with Leishmania Parasites
The study was carried out after being approved by the ethical committee of Shiraz University of Medical Sciences (SUMS, ethical reference No. IR.SUMS.REC.1394.S446). The animals were contained in condition with 12:12-hour dark-light cycle, 40-50% humidity, at 25±3°C temperature. Standard diet and free access to water were provided for the animals. Twenty four female BALB/C mice (four-week-old, weight about 18 grams) were obtained from Pasteur Institute, Tehran, Iran. Leishmania major (strain reference No: MRHO/IR/75/ER) was used in this study. Mice infection was done by using amastigote form of the parasite. To do this, 0.2 mL of a suspension containing 4*105 amastigotes (counted by a haemocytometer counting-chamber) was subcutaneously injected into the top of mice’s tail base by insulin syringe. Lesions were developed after about 3 weeks of inoculating of the parasites [Table/Fig-1]. Mice were divided into four groups, where the first group was treated with the extract of Arnebia euchroma (5% Arnebia solution; 5 g Arnebia in 100 mL of distilled water) and the second group with 5% gel of Arnebia euchroma (arnG) [17]. The third group was treated by meglumine antimoniate with a dose of 20 milligrams per kilogram. The negative control group received only normal saline. Treatments were started from the day open wounds were observed and carried out every 3 days for 21 days. The size of lesion on tail base was weekly measured. The length of the major and minor axes of the lesion at the base of the mouse’s tail perpendicular to each other were measured with a direct reading Vernier caliper and the area of the lesion in square millimeters was achieved by calculating ellipse area formula as originally described by Diaz K et al., [18].
BALB/c mice with tail lesions induced by L. major.
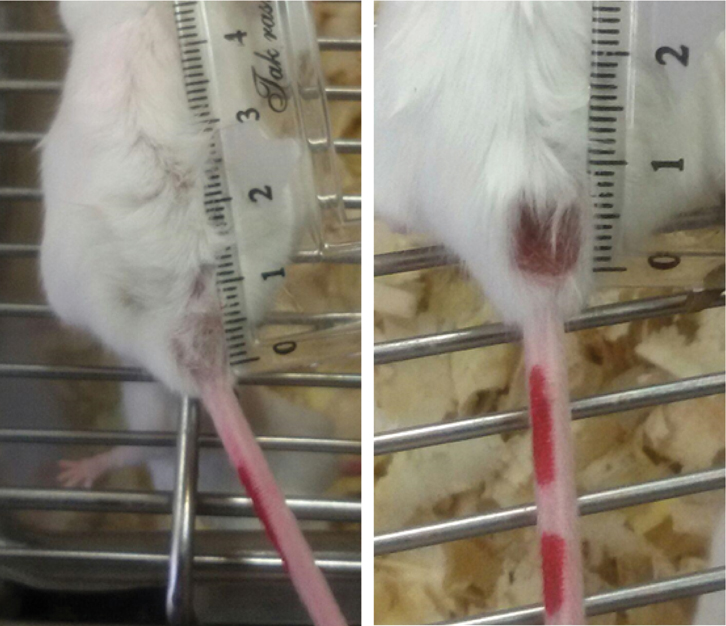
The animals were euthanized at the end of treatment. From the wound’s site, a circular skin sample along with 1 cm of the margin around the lesion area was removed and used for stereological assessments.
Stereological Study
A video-microscopy system made up of a microscope (E-200, Nikon™, Japan) linked to a video camera was used for the microscopic analysis of the skin samples. By using the 5 μm thickness slides and the point counting stereological method, at the magnification of 500X, the volume densities of the vessels, collagen bundles, and hair follicles were estimated. Moreover, the mean diameter of the vessels and also the vascular length density (Lv) were measured, as described by Mohsenikia M et al., [17].
The fibroblasts numerical density (Nv) (the number of the cells per unit volume of the dermis) were estimated by the 15 μm slides at a magnification of 1200× on the monitor, with the optical dissector stereological method.
Statistical Analysis
The data were collected, analysed and reported as the mean and standard deviation (mean±SD). Besides, the statistical comparisons between the groups were carried out by the SPSS software (v.17.0). Repeated measure ANOVA and One-way ANOVA tests were used in order to analyse and compare each parameter between the groups. p<0.05 was considered as statistically significant.
Results
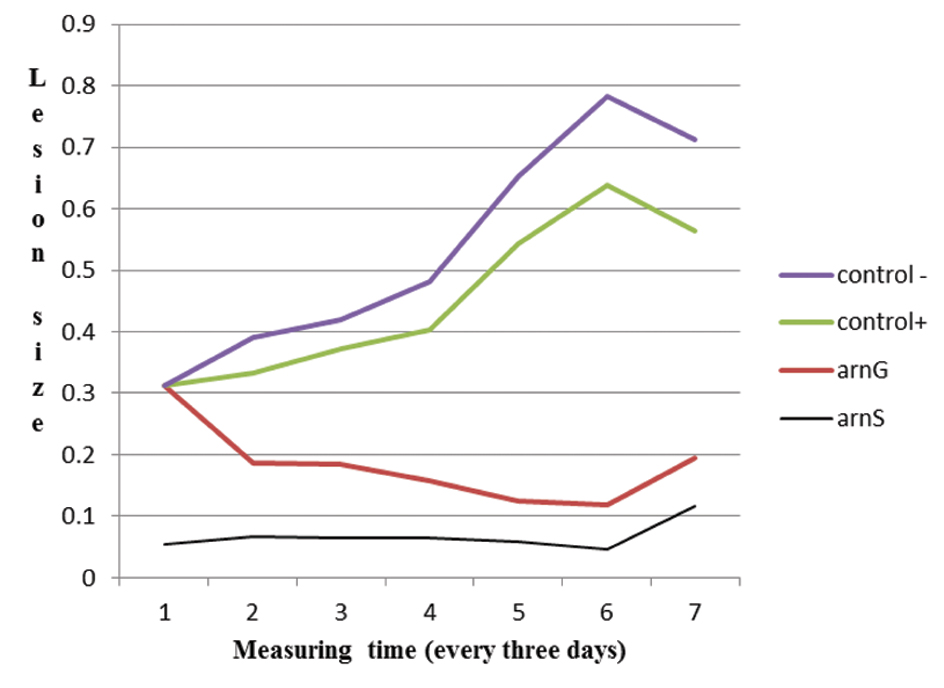
The mice group which received Arnebia extract showed faster lesion healing, although not statistically significant, in comparison to both positive and negative controls (p=0.611). Moreover, there was no significant difference between mice group receiving Arnebia extract with those receiving Arnebia 5% gel. [Table/Fig-2] shows the mean size of lesions in various periods of time in four groups of studied mice.
Mean size of lesions during three measuring sessions in different series of mice. Negative control (Control -) received normal saline; arnS: treated with 5% of the extract of Arnebia euchroma; arnG: treated with 5% gel of Arnebia euchroma; positive control (Control +) treated by meglumine antimoniate with a dose of 20 mg/kg.
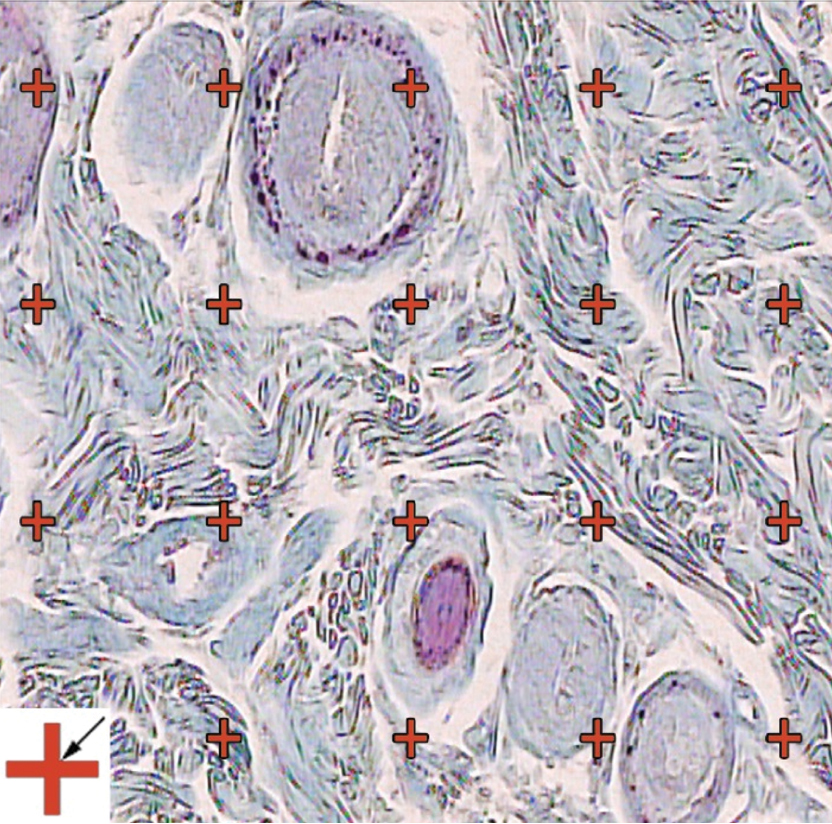
Stereological evaluations of the lesions showed degrees of inflammation, disorganisation, and ulcers in control as well as Arnebia-treated mice. However, there was a sign of the decrease in the histological grading’s including, ulceration, inflammation, and destruction, in treated mice, although non-significant [Table/Fig-3].
Stereological evaluations of the lesions. The volume density (Vv (collagen/dermis)) of the collagen fibers was estimated using a grid of points on the live image of dermis. (Hedenhain’s azan stain) (×450).
Discussion
Pentavalent antimony derivatives such as meglumine antimoniate are used currently to treat different forms of leishmaniasis [19]. Although pentavalent antimony derivatives are first-line therapy in the treatment of CL, however, drug failure and drug resistance are increasing in many regions of the world [13,19,20]. Miltefosine, as an anti-leishmanial drug, is a big hope for the researchers because it is administered orally and has significant efficacy but parasites become resistant to this hope-bringing drug too easily as a result, there is still a need to find better treatments [20].
Now-a-days researchers give more attention to herbal medicine due to lack of ideal drugs against Leishmania. Plant derivatives or extracts are appropriate candidates to offer a suitable source for the development of new medicinal agents for the treatment of leishmaniasis [21-24]. Artemisia species, Allium sativum, Achillea millefolium, Peganum harmala and Thymus vulgaris are the most herbal extract or derivatives which have been studied for their anti-leishmanial effects in Iran [22]. Among these plants, the IC50 values for extracts or derivatives of Allium spp, Alkanna spp, and Artemisia spp showed the significant difference in comparison with the rest of herbal extract, with the highest value for Allium spp [22].
Arnebia euchroma extract has been used as herbal medicine for skin disorders in Iran [25,26]. It has been shown that Arnebia euchroma extract has anti-inflammatory activities [27]. In our study, Arnebia euchroma did not show significant anti-leishmanial activities although there was a mildly (not significant) faster wound healing effect in mice which received either extract or gel of Arnebia in comparison with both negative and positive control groups. Same results have been reported for topical 10% and 20% gel of Echinacea purpurea extract which was not considered effective in the treatment or control of CL [24].
Limitation
Limitations of the study were including the bacterial infection, which is not uncommon in CL, in the tail base of mice which may reduce the efficacy of the Arnebia euchroma extract and cause slower wound healing than what we expected. Other limitations which can be stated might be using inappropriate, more or less, extract dosage of the extract. More studies are needed to find out the real anti-leishmanial effect of Arnebia euchroma.
Conclusion
Findings of this study revealed that administration of topical 5% gel of Arnebia euchroma extract does not have a considerable effect for treatment of L. major-induced CL in BALB/c mice.
Financial support and sponsorship: The study was financially supported by the office of vice-chancellor for research of Shiraz University of Medical Sciences (Grant No. 93-01-21-7882).
[1]. Sarkari B, Ahmadpour NB, Motazedian MH, Mirjalali H, Akhoundi M, Mohebali M, Inter-and intraspecific variations of Leishmania strains isolated from patients with cutaneous and visceral leishmaniases in Fars Province, South of IranIranian J Med Sci 2016 41(3):209-16. [Google Scholar]
[2]. Sarkari B, Gadami F, Shafiei R, Motazedian MH, Sedaghat F, Kasraian L, Seroprevalence of Leishmania infection among the healthy blood donors in kala-azar endemic areas of IranJ Parasit Dis 2015 39(3):545-49.10.1007/s12639-013-0393-326345068 [Google Scholar] [CrossRef] [PubMed]
[3]. Sarkari B, Hatam G, Ghatee M, Epidemiological features of visceral leishmaniasis in Fars province, southern IranIran J Public Health 2012 41(4):94-99. [Google Scholar]
[4]. Sarkari B, Naraki T, Ghatee MA, Abdolahi Khabisi S, Davami MH, Visceral leishmaniasis in Southwestern Iran: A retrospective clinico-hematological analysis of 380 consecutive hospitalized cases (1999-2014)PLoS One 2016 11(3):e015040610.1371/journal.pone.015040626942443 [Google Scholar] [CrossRef] [PubMed]
[5]. Shirzadi MR, Esfahania SB, Mohebalia M, Ershadia MR, Gharachorlo F, Razavia MR, Epidemiological status of leishmaniasis in the Islamic Republic of Iran, 1983-2012East Mediterr Health J 2015 21(10):736-42.10.26719/2015.21.10.73626750164 [Google Scholar] [CrossRef] [PubMed]
[6]. Sarkari B, Pedram N, Mohebali M, Moshfe AA, Zargar MA, Akhoundi B, Seroepidemiological study of visceral leishmaniasis in Booyerahmad district, south-west Islamic Republic of IranEast Mediterr Health J 2010 16(11):1133-36.10.26719/2010.16.11.113321218736 [Google Scholar] [CrossRef] [PubMed]
[7]. Davami MH, Motazedian MH, Sarkari B, The changing profile of cutaneous leishmaniasis in a focus of the disease in Jahrom district, southern IranAnn Trop Med Parasitol 2010 104(5):377-82.10.1179/136485910X1278638989108320819305 [Google Scholar] [CrossRef] [PubMed]
[8]. Sarkari B, Bavarsad Ahmadpour N, Moshfe A, Hajjaran H, Molecular evaluation of a case of visceral leishmaniasis due to leishmania tropica in Southwestern IranIranian J Parasitol 2016 11(1):126-30. [Google Scholar]
[9]. Sarkari B, Hatam GR, Adnani SJ, Asgari Q, Seroprevalence of feline leishmaniasis in areas of Iran where Leishmania infantum is endemicAnnals of Tropical Medicine And Parasitology 2009 103(3):275-77.10.1179/136485909X39827619341541 [Google Scholar] [CrossRef] [PubMed]
[10]. Pourmohammadi B, Motazedian M, Hatam G, Kalantari M, Habibi P, Sarkari B, Comparison of three methods for diagnosis of cutaneous leishmaniasisIran J Parasitol 2010 5(4):1-8. [Google Scholar]
[11]. Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, López-Vélez R, García-Hernández R, Drug resistance and treatment failure in leishmaniasis: A 21st century challengePLoS Negl Trop Dis 2017 11(12):e000605210.1371/journal.pntd.000605229240765 [Google Scholar] [CrossRef] [PubMed]
[12]. Singh K, Garg G, Ali V, Current therapeutics, their problems and thiol metabolism as potential drug targets in leishmaniasisPLoS Negl Trop Dis 2016 17(9):897-919.10.2174/138920021766616081916144427549807 [Google Scholar] [CrossRef] [PubMed]
[13]. Pourmohammadi B, Motazedian MH, Handjani F, Hatam GH, Habibi S, Sarkari B, Glucantime efficacy in the treatment of zoonotic cutaneous leishmaniasisSoutheast Asian J Trop Med Public Health 2011 42(3):502-08. [Google Scholar]
[14]. Sharma R, Singh B, Singh D, Ethnomedicinal, pharmacological properties and chemistry of some medicinal plants of Boraginaceae in IndiaJ Med Plant Res 2009 3(13):1153-75. [Google Scholar]
[15]. Kaith B, Kaith N, Chauhan N, Anti-inflammatory effect of Arnebia euchroma root extracts in ratsJ Ethnopharmacol 1996 55(1):77-80.10.1016/S0378-8741(96)01477-8 [Google Scholar] [CrossRef]
[16]. Chen X, Yang L, Zhang N, Turpin JA, Buckheit RW, Osterling C, Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1Antimicrob Agents Chemother 2003 47(9):2810-16.10.1128/AAC.47.9.2810-2816.200312936978 [Google Scholar] [CrossRef] [PubMed]
[17]. Mohsenikia M, Khakpour S, Azizian Z, Ashkani-Esfahani S, Razavipour ST, Toghiani P, Wound Healing Effect of Arnebia euchroma gel on Excisional Wounds in RatsAdv Biomed Res 2017 6:210.4103/2277-9175.19926028217647 [Google Scholar] [CrossRef] [PubMed]
[18]. Diaz K, Castañeda B, Miranda C, Lavarello R, Llanos A, Development of an acquisition protocol and a segmentation algortihm for wounds of cutaneous Leishmaniasis in digital imagesIn Medical Imaging 2010 :762310.1117/12.844534 [Google Scholar] [CrossRef]
[19]. Ouellette M, Drummelsmith J, Papadopoulou B, Leishmaniasis: drugs in the clinic, resistance and new developmentsDrug Resist Updat 2004 7(4):257-66.10.1016/j.drup.2004.07.00215533763 [Google Scholar] [CrossRef] [PubMed]
[20]. Croft SL, Sundar S, Fairlamb AH, Drug resistance in leishmaniasisClin Microbiol Rev 2006 19(1):111-26.10.1128/CMR.19.1.111-126.200616418526 [Google Scholar] [CrossRef] [PubMed]
[21]. Moghaddas E, Khamesipour A, Mohebali M, Fata A, Iranian native plants on treatment of cutaneous leishmaniosis: a narrative reviewIranian J Parasitol 2017 12(3):312-22. [Google Scholar]
[22]. Soosaraei M, Fakhar M, Hosseini Teshnizi S, Ziaei Hezarjaribi H, Banimostafavi ES, Medicinal plants with promising antileishmanial activity in Iran: a systematic review and meta-analysisAnn Med Surg (Lond) 2017 21:63-80.10.1016/j.amsu.2017.07.05728794869 [Google Scholar] [CrossRef] [PubMed]
[23]. Sarkari B, Mohseni M, Moein MR, Shahriarirad R, Asgari Q, Effect of hydroalcoholic extract of Echinacea purpurea in combination with meglumine antimoniate on treatment of Leishmania major-induced cutaneous leishmaniasis in BALB/c miceInt J Appl Basic Med Res 2017 7(1):53-56.10.4103/2229-516X.19852428251109 [Google Scholar] [CrossRef] [PubMed]
[24]. Sarkari B, Sattari H, Moein MR, Tamadon AM, Rad RS, Asgari Q, Effect of topical gel prepared with hydroalcoholic extract of Echinacea purpurea on treatment of Leishmania major-induced cutaneous leishmaniasis in BALB/C miceJ Pharm Negat Results 2016 7(1):1210.4103/0976-9234.177054 [Google Scholar] [CrossRef]
[25]. Hosein Farzaei M, Abbasabadi Z, Reza Shams-Ardekani M, Abdollahi M, Rahimi R, A comprehensive review of plants and their active constituents with wound healing activity in traditional Iranian medicineWounds 2014 26(7):197-206. [Google Scholar]
[26]. Nasiri E, Hosseinimehr SJ, Zaghi Hosseinzadeh A, Azadbakht M, Akbari J, The effects of Arnebia euchroma ointment on second-degree burn wounds: a randomized clinical trialJ Ethnopharmacol 2016 189:107-16.10.1016/j.jep.2016.05.02927180881 [Google Scholar] [CrossRef] [PubMed]
[27]. Zhang ZL, Fan HY, Yang MY, Zhang ZK, Liu K, Therapeutic effect of a hydroxynaphthoquinone fraction on dextran sulfate sodium-induced ulcerative colitisWorld J Gastroenterol 2014 20(41):15310-18.10.3748/wjg.v20.i41.1531025386079 [Google Scholar] [CrossRef] [PubMed]