Chronic obstructive pulmonary disease is one of the leading cause of morbidity and mortality worldwide and results in an economical and social burden that is both substantial and in increasing trend [1]. According to World Health Organisation, it has been estimated that around 65 million people have moderate to severe COPD [2]. In 2002, COPD was the fifth leading cause of death but now it is fourth and will become third leading cause of death till 2030 worldwide [2]. In India, out of all non-communicable diseases, chronic respiratory disease accounted for 7% deaths and 3% DALYs lost [3]. It has been estimated that there are 30 million COPD patients in India and out of which 556,000 die annually [4,5]. Based on the ‘Indian Study of Asthma, Respiratory Symptoms and Chronic Bronchitis’ (INSEARCH) study, the prevalence of chronic bronchitis is 3.49% (4.29% in males and 2.7% in females) in adults > 35 years [6].
The spectrum of CVDs are manifested as sequelae and includes right ventricular dysfunction, pulmonary hypertension, coronary artery disease, left ventricular dysfunction and arrhythmias [7]. When pulmonary vascular disease is associated with COPD, there is increased morbidity and it worsens survival [8]. Patients with COPD also carry an increased risk of mortality due to arrhythmia, myocardial infarction or congestive cardiac failure compared to those who do not [8]. Based on previous study, cardiovascular complication is one of the important cause of mortality even in mild COPD patients [9].
The prevalence of cardiovascular diseases among COPD patients depends on type of cardiovascular abnormality which varies based on the results of different studies i.e., pulmonary hypertension (ranges from 19% to 63%), Cor-pulmonale (ranges from 11.6% to 41.7%), Left ventricular systolic dysfunction (ranges from 6% to 13%), Left ventricular diastolic dysfunction (ranges from 12% to 58%) [10-14]. Some studies pointed out that there is a linear correlation between pulmonary hypertension verses severity of COPD and Cor-pulmonale verses severity of COPD [10-13]. In the INSEARCH study, based on questionnaires, prevalence of chronic bronchitis at one center of Orissa was 1.92% [6]. Therefore, the present study aimed to identify cardiovascular changes in established COPD patients and to find out the correlation between the cardiac abnormality and the severity of COPD.
Materials and Methods
The present study was conducted in the Outpatient Department of Pulmonary Medicine of Veer Surendra Sai Medical College, Burla, Odisha, India; from September 2014 to August 2016. Informed consent was taken from all participants of the present study. The research protocol was approved by the Institutional Ethics Committee. Patient’s socio-economical status was classified according to modified Kuppuswami socioeconomic status scale [15]. Patients were diagnosed with COPD based on symptoms, history of exposure to risk factors (tobacco smoke, occupational dusts and chemicals, smoke from home cooking and heating fuel), clinical examination and spirometry (post bronchodilator FEV1/FVC <70%). Patients were classified based on GOLD staging [Table/Fig-1]. The patients with history of Pulmonary tuberculosis, Bronchial Asthma, Bronchiectasis, Bronchiolitis Obliterans, Interstitial Lung Disease, Hypertension, other vascular disorders, known Congenital and Acquired Heart disease and those unable to perform spirometry were excluded from this study.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) staging.
| COPD GOLD Stage | FEV1/FVC | FEV1 percentage of predicted |
|---|
| I (Mild) | <70% | ≥80% |
| II (Moderate) | <70% | <80% but ≥50% |
| III (Severe) | <70% | <50% but ≥30% |
| IV (Very severe) | <70% | <30% |
COPD: Chronic obstructive pulmonary disease; GOLD: Global initiative for chronic obstructive lung disease
Procedure of maneuver, acceptability criteria, reproducibility criteria and recording criteria of spirometry was done based on ATS guideline [16]. Diagnosed COPD patients, fulfilling the inclusion criteria were included within the above time period by convenient sampling method. All enrolled patients underwent Chest X-ray PA view, ECG and Echocardiography. Chest X-ray was analysed and a consensus reached by at least two experts, out of which, one was a radiologist and the other was a chest physician. Radiographic findings were noted based on the type of lesion (low and flat diaphragm, tubular heart, increased diameter of pulmonary artery, increased translucency, cardiothoracic ratio >50%).
Toshiba Echo machine with 4 MHz transducer was used and echo was performed by trained cardiologist. Echocardiography was performed to assess the pericardium, valvular anatomy and function, left and right side chamber size and cardiac function. Measurements were made by M-mode, 2-D mode, colour flow mapping, and pulsed and continuous wave Doppler recordings were obtained for each subject. Parameters recorded were right ventricular dilation, Left ventricular hypertrophy, Left ventricular ejection fraction, fractional shortening, LV Diastolic function, trans tricuspid pressure gradient. Right ventricular systolic pressure was considered to be equal to the Systolic Pulmonary Artery Pressure (SPAP) in the absence of right ventricular outflow obstruction. Right ventricular systolic pressure was equal to sum of Trans-Tricuspid Pressure Gradient (TTPG) and Right Atrial Pressure (RAP). TTPG was calculated based on modified Bernoulli equation i.e., 4v2 (v=peak velocity of tricuspid regurgitation, m/s) [17]. RAP was estimated to be 5, 10, or 15 mmHg based on the variation in the size of inferior vena cava with inspiration i.e. complete collapse, RAP=5 mmHg; partial collapse, RAP=10 mmHg; and no collapse, RAP=15 mmHg [18].
Mean Pulmonary Artery Pressure (MPAP) was calculated by using Chemla formula i.e., 0.61 SPAP+2 mmHg [19]. Left ventricular ejection fraction <50% was considered as low ejection fraction. Pulmonary Hypertension (PH) was classified based on MPAP. Mild PH was defined as MPAP within 25 to 35 mmHg, Moderate PH at a pressure of 36-45 mmHg and Severe PH at pressure limit exceeding more than 45 mmHg. Cor-pulmonale was defined as alteration of structure and function of right ventricle such as right ventricular dilatation. Left ventricular fractional shortening <25% was considered as low fractional shortening [20].
ECG interpretation and echocardiogram were done by two different persons to reduce the bias.
Statistical Analysis
The statistical analysis was performed with the help of SPSS version 22.0 (IBM SPSS Statistics, IBM Corporation). Normally distributed continuous variables are presented as mean±SD and non-normally distributed continuous variables are presented as median with interquartile range. Categorical variables were expressed as percentage. Different variables were tested; by chi-square test in case of comparison of nominal variables, Fisher’s-Exact test for comparison of nominal variable of small sample size, and for more than two independent non-parametric variable by Kruskal-Wallis test. A p-value of 0.05 or less was considered statistically significant.
Results
Eighty COPD patients were included in this study. Patient characteristics are shown in [Table/Fig-2].
Baseline characteristic and demographic profile.
| Variable | Male (n=60) | Female (n=20) |
|---|
| Age | 50-59 years | 6 | 6 |
| 60-69 years | 40 | 10 |
| >70 years | 14 | 4 |
| 67.5 (5)[median (interquartile range)] | 65.2±6. 50 [mean(SD)] |
| Socioeconomical status | Upper | 0 | 0 |
| Upper middle | 6 | 0 |
| Lower middle | 0 | 6 |
| Upper lower | 54 | 14 |
| Lower | 0 | 0 |
| Occupation | Farmer | 44 | 2 |
| Daily labourer | 10 | 6 |
| Housewife | 0 | 12 |
| Government service | 6 | 0 |
| Smoker | 58 | 6 |
On the basis of GOLD classification, there were two patients in Stage I, 34 patients in Stage II, 28 patients in Stage III and 16 patients in Stage IV. Breathlessness score was recorded based on modified Medical Research Council (mMRC) [21]. All patients of GOLD Stage I, II and IV had mMRC dyspnea score of 2, 2 and 4 respectively. However, majority (71%) patients of GOLD Stage III had mMRC dyspnea score 3 at the time of enrolment.
Median (interquartile range) duration of symptoms in GOLD Stage II, III and IV group were 5 (4), 15 (3) and 15 (7) in years respectively. Patient having higher grade of COPD had progressively longer duration of symptoms which was found to be statistically significant by Kruskal-Wallis test.
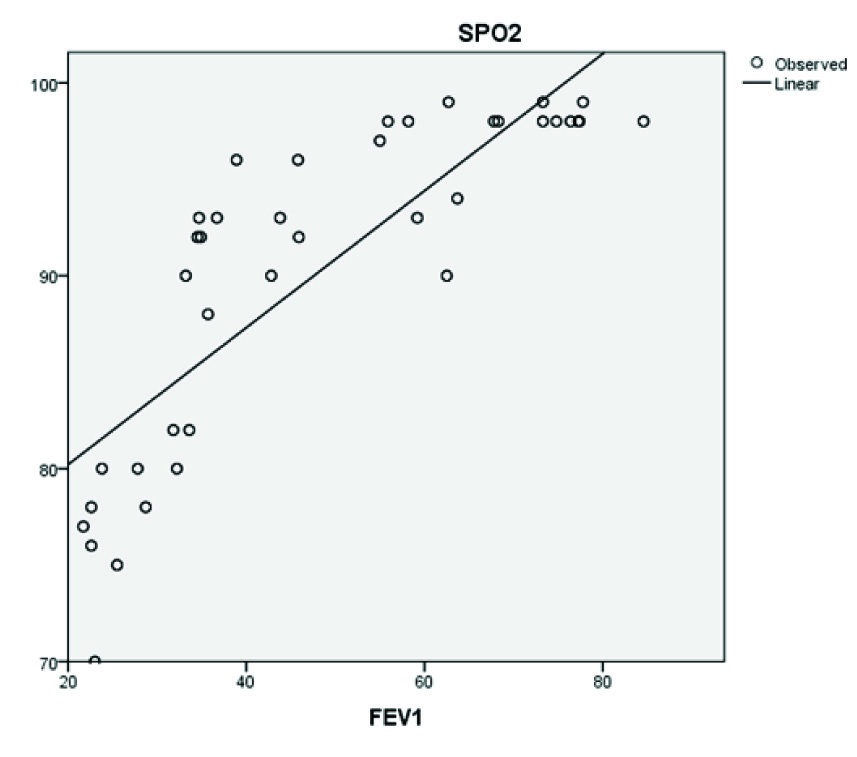
Oxygen saturation by Pulse Oxymetry (SPO2) was noted in all patients. Hypoxemia was defined as a patient having SPO2<95% on room air. None of the patients of GOLD Stage I had hypoxemia but six patients (18%) of GOLD Stage II, 24 patients (86%) of GOLD Stage III and 16 patients (100%) of GOLD Stage IV were hypoxemic. Relationship between FEV1 and SPO2 was positive linear as showed in scatter diagram [Table/Fig-3].
Scatter diagram showing positive linear correlation of SPO2 and FEV1.
Among all, 25% of patients had a normal chest X-ray. The most common radiological finding was increased translucency (75%) followed by low and flat diaphragm and tubular heart (67.5%). A total of 16.3% patients had evidence of increased diameter of pulmonary artery on X-ray chest. Majority of the radiographic changes were seen in GOLD Stage III and IV. Chest radiography was normal in all cases of GOLD Stage I. Features of hyperinflation like increased translucency, low and flat diaphragm and tubular heart were seen more frequently in the GOLD Stage III and IV. Features of cardiovascular abnormalities like; increased diameter of pulmonary artery and cardiothoracic ratio >50% was seen in GOLD Stage III and IV groups. Increased diameter of pulmonary artery was more in GOLD stage IV than GOLD Stage III, which was statistically significant (p<0.001, Fisher’s-Exact test).
The most common ECG change was Sinus Tachycardia (37.5%), followed by poor progression of R wave (25%). Right axis deviation was seen in 15% of cases and P-pulmonale was seen in 17.5%. Right ventricular hypertrophy and low voltage QRS complex were seen in 17.5% and 12.5% of cases, respectively.
There was no ECG abnormality in both GOLD I and II groups [Table/Fig-4]. Features of right heart pathology like; P pulmonale, right axis deviation and right ventricular hypertrophy were more seen in GOLD IV group than GOLD III groups which was also statistically significant (p<0.05). Features of hyperinflation like low voltage QRS complex were more seen in GOLD Stage IV patients than GOLD III group which was also statistically significant (p<0.05). Tachycardia and poor R wave progression were more seen in GOLD Stage III than Stage IV however not significant (p>0.05).
Association GOLD stages with ECG changes and Echocardiography finding.
| ECG change | Stage I (n=2) (%) | Stage II (n=34) (%) | Stage III (n=28) (%) | Stage IV (n=16) (%) | p-value (Chi-square test/Fisher’s Exact test) |
|---|
| Tachycardia (30) | 0 | 0 | 20 (71.43%) | 12 (75%) | 1 |
| P pulmonale (14) | 0 | 0 | 4 (14.28%) | 10 (62.5%) | 0.001£ |
| Low amplitude QRS (10) | 0 | 0 | 2 (7.14%) | 8 (50%) | 0.002 |
| RAD (12) | 0 | 0 | 2 (7.14%) | 8 (50%) | 0.002 |
| Poor R wave progression (20) | 0 | 0 | 12 (42.85%) | 8 (50%) | 0.647£ |
| RVH (14) | 0 | 0 | 4 (14.28%) | 10 (62.5%) | 0.001£ |
| Echocardiography finding |
| RV dilatation (n=24) | 0 | 0 | 10 (35.71%) | 14 (87.5%) | 0.001£ |
| LVH (n=10) | 0 | 0 | 2 (7.14%) | 8 (50%) | 0.002 |
| Low EF (n=8) | 0 | 0 | 0 | 8 (50%) | @ |
| Low FS (n=8) | 0 | 0 | 0 | 8 (50%) | @ |
| LV diastolic dysfunction (n=14) | 0 | 0 | 4 (14.28%) | 10 (62.5%) | 0.001£ |
| PH (n=26) | 0 | 2 (5.8%) | 12 (42.85%) | 12 (75%) | <0.00001μ |
£Comparison between GOLD Stage III and IV; μcomparison between GOLD II, III and IV; @Statistical test not done.
RAD: Right axis deviation; RVH: Right ventricular hypertrophy; RV: Right ventricle, LVH: Left ventricular hypertrophy; EF: Ejection fraction, FS: Fractional shortening, PH: Pulmonary hypertension
Most common echo-cardiographic abnormality noticed was PH (32.5%) followed by RV Dilatation (30%). Around 43.75% of patient’s echocardiography finding was normal. Right ventricular dilatation, LV hypertrophy, LV diastolic dysfunction and PH was more in GOLD Stage IV than Stage III, which was statistically significant (p<0.05).
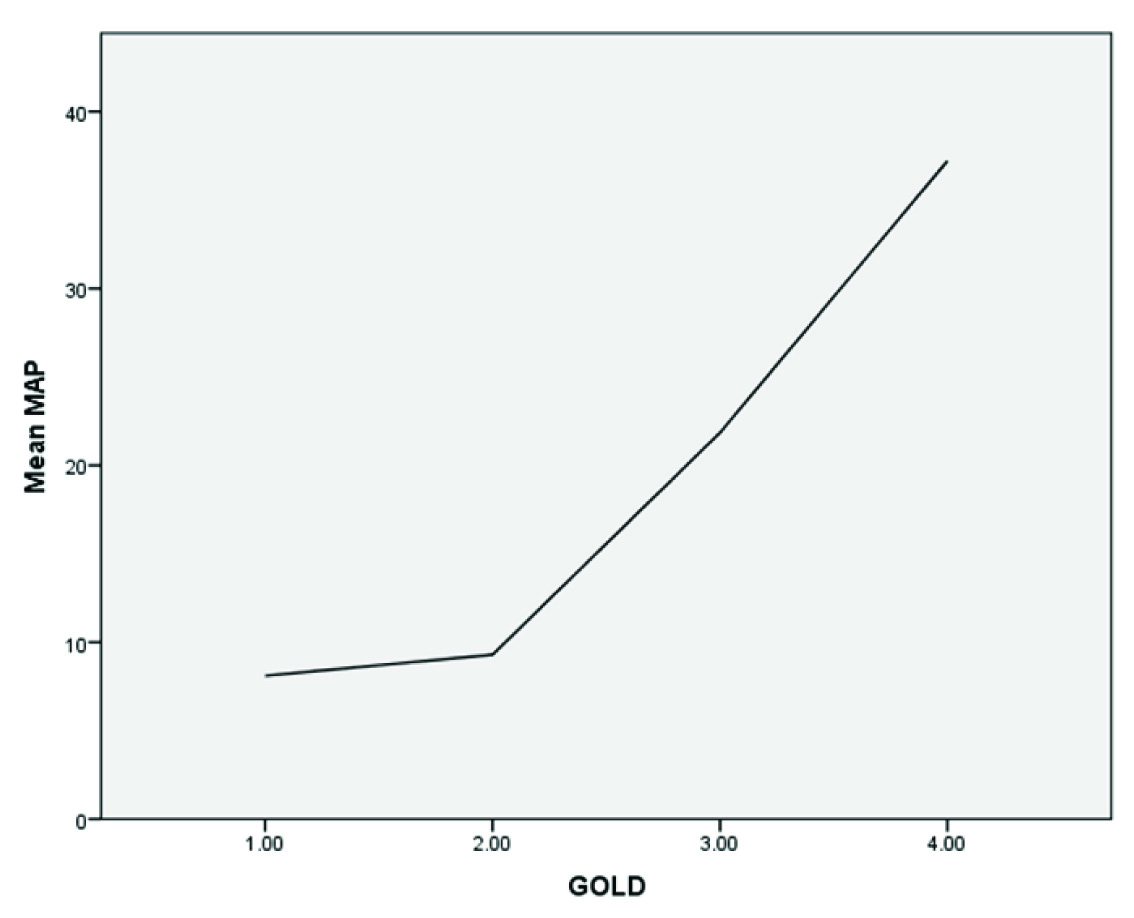
Pulmonary Hypertension was observed in 26 patients i.e., 32.5% of total study population [Table/Fig-5]. Among all PH, 15.38% of patients were having mild, 38.4% and 46% having moderate and severe PH respectively. The patients having PH in GOLD Stage II, were mild grade. Prevalence of severe PH was more in GOLD IV compared to GOLD III (Fisher’s-Exact test, p-0.016. Odds ratio>1 along with 95% confidence interval) which was statistically significant. There is a direct relationship between mean pulmonary arterial pressure and stages of GOLD [Table/Fig-6].
Relation of severity of pulmonary hypertension with stages of GOLD.
| Severity of pulmonary hypertension | Stage I (n=2) (%) | Stage II (n=34) (%) | Stage III (n=28) (%) | Stage IV (n=16) (%) |
|---|
| Mild(MPAP 25-35 mmHg)(n=4) | 0 | 2 (5.9%) | 2 (7.1%) | 0 |
| Moderate(MPAP 36-45 mmHg)(n=10) | 0 | 0 | 6 (21.4%) | 4 (25%) |
| Severe(MPAP>45 mmHg)(n=12) | 0 | 0 | 4 (14%) | 8 (50%) |
Graph showing relationship between MAP and GOLD severity. There is steep increase of MAP at GOLD III and IV.
Discussion
It has been demonstrated by various studies that, level of pulmonary hypertension is a prognostic indicator in COPD. Frequency of PH in COPD ranges from 19% to 63% in various studies whereas in the present study it was 32.5% [10-14]. In the present study, frequencies of PH was more in severe degree of COPD and similar finding have been observed by Gupta NK et al., and Maula F et al., [10,22]. Mean pulmonary arterial pressures have moderately positive co-relation with the severity of stages of GOLD in the present study, which indicates that as the COPD progresses the chances of increased pulmonary arterial pressure are more but this progression is faster towards the GOLD Stage IV. Previous studies showed the frequencies of severe PH in COPD ranges from 2.8% to 25% [10-14], however in present study it was 15%. In the present study except mild PH; rest of PH patients was hypoxemic. In the study, an inverse association between GOLD stage and SpO2 was seen. As COPD progresses to more severe grade, the chances of hypoxia are more.
Cor-pulmonale was seen in 30% of patients but Gupta NK et al., and Maula F et al., found it in 17.5% and 32.4% of COPD patients, respectively and they did not find any correlation between cor-pulmonale and severity of COPD [10,22].
Right sided cardiac pathology is a well known complication in COPD, due to the backpressure from pulmonary vasculature. However, evidence from a recent study shows that left sided pathology is also possible due to COPD [10]. The presence of abnormal left ventricular performance could be due to COPD or due to the association of other sub-clinical or clinical cardiac diseases. Possible explanations are concurrent coronary artery diseases/atherosclerosis, bulging of ventricular septum towards left due to dilatation of right ventricle (ventricular interdependence), chronic hypoxemia which leads to myocardial relaxation abnormality, lung hyperinflation leading to increased stiffness of the parietal pleura and combined effect leading to added load on ventricle [23]. In the present study, LV Diastolic Dysfunction was seen in 17.5% out of the total study population. In the present study, all the patients having diastolic dysfunction have concomitant moderate or severe PH and hypoxia. However, one study showed, LV diastolic dysfunction may be present in COPD patients with normal pulmonary arterial pressure [24]. LV systolic dysfunction was seen in 10% in present study, and found only seen in GOLD Stage IV patient and the majority was associated with severe PH, hypoxia with left diastolic dysfunction, left ventricular hypertrophy, right ventricular hypertrophy and dilatation. Study by Gupta NK et al., LV systolic dysfunction was 7.5% and LV diastolic dysfunction was 47.5% [10]. Similar findings were observed by Yilmaz R et al., in the absence of pre-existing cardiovascular pathology (ischaemic heart disease, systemic arterial hypertension, etc.,), the systolic function abnormality due to COPD was rarely found, usually in severe pulmonary hypertension and in patients with right ventricular dysfunction [25].
Left ventricular hypertrophy was seen in 12.5% in the present study. Frequency of LVH was seen more in very severe COPD as compared to severe COPD. Study by Gupta NK showed LVH in 22.5% of patients, while a study by Anderson WJ et al., showed LVH in 21.4% in men and 43.2% in women with normoxemic COPD without PH [10,26]. The author proposed that sympathetic activation mainly through renin-angiotensin-aldosterone system is the possible mechanism [26]. In the present study, all patients had left ventricular pathology like LVH, left ventricular systolic dysfunction and left ventricular diastolic dysfunction; patients were associated with hypoxia, so probably hypoxia may be a trigger factor for sympathetic activation and myocardial relaxation abnormality.
Chest radiographic abnormalities tend to be more frequent in more severe degrees of COPD. Increased diameter of pulmonary artery and cardiothoracic ratio were two variables on X-ray chest, which indicate the cardiovascular abnormality. Both findings were more seen in GOLD Stage IV COPD.
Prevalence of P-pulmonale ranged from 15.5% to 35.7% in different studies [27-29]; however, in the present study it was 17.5%. In this study, P pulmonale, low QRS complex, RAD and RVH were seen with higher frequency in very severe COPD compared to severe COPD. According to Larssen MS et al., increased airways obstruction and RV after load were associated with RV mass and clockwise rotation of horizontal QRS axis, whereas emphysema was associated with increased heart rate and reduced QRS amplitude [30].
Limitation
There were also some limitations of the present study like arterial blood gas analysis was not done for which PaCO2 value could not be analysed which could have shown certain correlation among different electrocardiography and echocardiography variables. Pulmonary artery pressure measurement by pulmonary artery catheter was not done, which is gold standard for the diagnosis of PH. Certain confounding factors like co-morbidity or pack cell volume that may be associated with the cardiovascular morbidity which were not analysed. As the present study was a cross-sectional study, we were not able to differentiate whether cardiovascular abnormalities were due to COPD or co-morbidity. A well designed prospective study may give the answer.
Conclusion
The frequency of X-ray chest changes and ECG changes are proportionate to the degree of severity of COPD. Prevalence of different cardiovascular diseases are PH 32.5%, left ventricular systolic dysfunction 10%, left ventricular diastolic dysfunction 17.5% and Cor-pulmonale 30% in the present study. The present study findings are little different than previous published Indian studies. The frequency as well as the severity of PH increases as the severity of COPD increases. The frequency of Cor-pulmonale increases as the severity of COPD increases. COPD affects, not only the right ventricle, but also the left ventricle. Left ventricular systolic and diastolic dysfunction and left ventricular hypertrophy are also noted especially in severe and very severe COPD, in the absence of pre-existing heart disease or coronary artery disease. So screening of all COPD patients for cardiac abnormalities are suggested.
COPD: Chronic obstructive pulmonary disease; GOLD: Global initiative for chronic obstructive lung disease
£Comparison between GOLD Stage III and IV; μcomparison between GOLD II, III and IV; @Statistical test not done.
RAD: Right axis deviation; RVH: Right ventricular hypertrophy; RV: Right ventricle, LVH: Left ventricular hypertrophy; EF: Ejection fraction, FS: Fractional shortening, PH: Pulmonary hypertension