Gradual visual field loss as a result of retinal ganglion cell atrophy and optic neuropathy are the characteristic of Glaucoma. Most cases of POAG are asymptomatic but it may present as visual field defect. POAG is the most prevalent form of glaucoma with almost 45 million cases worldwide. This is expected to increase to 58.5 million by 2020. According to World Health Organization (WHO), it is the second most common cause of avoidable blindness after un-operated cataract and third most common cause of visual impairment worldwide. Global WHO data indicate that, glaucoma alone is responsible for about 5 million cases out of the total 37 million people who are currently blind. Glaucoma is projected to affect approximately 79 million people by 2020 [1-5].
Elevated IOP is a leading risk factor for the development of primary open angle glaucoma, and reducing IOP is shown to be imperative to slow disease progression in this disease. Medications are the first line of therapy in the management of glaucoma. This is mostly due to easier accessibility and having similar outcomes as compared to surgery. Medications for the treatment of glaucoma include β-blockers, α2 adrenergic, carbonic anhydrase inhibitors and PGA [6-9].
Prostaglandin analogues showed advantages over other medical treatments and are presently the initial medication of choice. They work primarily by increasing the uveoscleral outflow. PGAs are able to decrease IOP by 33%, which is more effective than other group of drugs. There is a higher rate of adherence to treatment as these drugs require only once a day dosing. The pressure lowering effect can last up to two days. They have short half-life which reduces the risk of systemic side effects. Latanoprost is the most frequently used PGA in clinical practice.
PGAs (latanoprost and travoprost) are typically administered in multi-dose bottles that contain preservatives to ensure sterility. Benzalkonium chloride (BAK, quaternary ammonium compound) is the most commonly used preservative in ophthalmic medications. BAK has broad-spectrum bactericidal and bacteriostatic activity at physiological pH. The concentration of BAK ranges from 0.004% to 0.02% in different ophthalmic solutions. At these concentrations, patients using medication with frequent dosing may be at risk of experiencing the adverse effects of BAK. With chronic, long-term exposure, BAK is reported to lessen the integrity of epithelial cells, increase the number of conjunctival inflammatory cells, causing a loss of goblet cells, reducing tear function and decrease in the tear film breakup time [10-12].
Global reports have suggested that BAK free preparations presents with fewer adverse reactions as compared to BAK containing preparations. There are other alternative preservatives available which are designed to eliminate the toxic side effects of BAK in multidose preparations [13-16].
Currently, preservative-free topical glaucoma medications which come in single-dose units are also available, and their use allows total avoidance of preservatives with all their associated adverse effects. However, single-dose units and preparations with alternative preservative, manufacturing and packaging make this type of medications expensive and difficult to use for some patients. Preservative free or alternative preservatives containing topical ophthalmic medications are recommended for the diseases, which required long-term treatment. Use of preservative free topical medications help in maintaining healthy ocular surface in long run treatment. But presence of preservatives such as BAK were a necessary evil for preventing bacterial contamination of eye drops, so the majority of glaucoma medications still contain some levels of BAK [17].
There is limited comparative study on BAK containing and preservative free prostaglandin analogues on Indian population, therefore the goal of the current study was to compare the efficacy, safety and tolerability of BAK containing and preservative free prostaglandin analogue latanoprost in patients with POAG.
Materials and Methods
This was a prospective study performed in collaboration of the ophthalmology departments of Moti Lal Nehru Medical College, George Town, Allahabad, Uttar Pradesh and approved by the Institutional Ethics Committee. The patients included in the study signed a written informed consent.
Patients: Study was carried out on the patients who attended outpatient department and glaucoma clinic at Regional Institute of Ophthalmology (RIO), M.D. Eye Hospital, Allahabad, Uttar Pradesh. Adult patients of either sex were screened on the basis of selection criteria and those fulfilling the criteria were included in the study.
Inclusion criteria: Patients who had given written informed consent. It included both men and women aged ≥18 years with a diagnosed case of primary open angle glaucoma. Patients were able to understand and follow study related advice.
Exclusion criteria: Patients not willing to give consent, patients with hypersensitivity with prostaglandin analogues or unable to discontinue all other IOP lowering ocular medications were not included in the study. Patients with any ocular inflammation or pathology or clinically significant retinal disease were also excluded from the study. Any intraocular surgery, intraocular trauma within six months and intraocular laser surgery within three months also make them unfit for the study. Patients with single functioning eye, or having abnormality that prevent them from ocular examination, modified Shaffer angle grade<2 in either eye, cup to disc ratio >0.8, severe central visual field loss and mean IOP>36 mm Hg in either eye at any time point were also excluded.
Study instruments: Topical ophthalmic solution of latanoprost, slit lamp biomicroscope and direct ophthalmoscope.
Methodology
At the baseline visit, complete ophthalmic examination including Best Corrected Visual Acuity (BCVA), Hyperemia Score, Tear Breakup Time (TBUT), Goldmann applanation tonometry, slit lamp biomicroscopy of the anterior eye segment and binocular indirect slit lamp fundoscopy was completed and those fulfilling the criteria were enrolled for the study.
Enrolled patients were started anti-glaucoma treatment after assigning group. Patients assigned in Group1 were treated with BAK preserved latanoprost 0.005% (LATOPROST contains 0.005% latanoprost, preservative: benzalkonium chloride 0.02%; SUN pharma laboratories Ltd. Mumbai) and patients in Group 2 were treated with BAK free latanoprost 0.005% (LACOMA contains 0.005% latanoprost, Ajanta pharma Ltd., Mumbai).
Study was done in two sequential phases that is screening/eligibility phase and treatment/follow up phase. Five visits were scheduled for treatment/follow up phase at day 1, day 14, day 30, day 42 and day 84.
All the patients were screened according to inclusion and exclusion criteria. If the patients were taking some topical medication before screening visit then it was advised to such patients to discontinue all medication up to predetermined washout period. If discontinuation of medication was not possible then such patients were not included in the study.
Eligible patients were randomised (by envelope method) in a 1:1 ratio. Patients were told to instill one drop of their assigned drug in both eyes once a day daily in the evening for the periods of 3 months, except if the patients safety issue were compromised. Patients were also instructed for follow up visits at their scheduled time interval. One eye from every patient was chosen as the investigation eye as per the criteria below; in the event that the two eyes were treated, the worse evaluable eye was chosen as the investigation eye (The worse eye was characterized as the eye with the higher IOP at 11 AM over the screening visit). In the event that IOP values were equivalent at 11 AM, the worse eye was chosen as the investigation eye with the higher IOP at 4 PM over the screening visit. And if the two eyes were equivalent at 4 PM, the right eye was chosen for investigation. At last, in the event that exclusively one eye of a patient was treated, that eye was chosen as the examination eye.
At subsequent visits study parameters (slit lamp biomicroscopy of the anterior eye segment, TBUT and Goldmann applanation tonometry) were assessed.
Hyperemia: Bulbar conjunctival hyperemia observations were graded by a comparison with colour photographic standards employing the following values: 0 = none (normal); 0.5 = trace (trace flush, reddish pink); 1 = mild (mild flush, reddish colour); 2 = moderate (bright red colour); and 3 = severe (deep, bright diffuse redness) [18].
Tear Break-Up Time (TBUT): To assess tear film instability and dry eye we had procured TBUT values in triplicate manner for each patient. TBUT values were obtained by placing 5 μL of 2% preservative free sodium fluorescent dye to the inferior fornix and patients were asked to blink at least three times for proper mixing of the dye. The timer was started just after last blink and patient was instructed not to blink again, then TBUT is recorded as the number of seconds that elapse between the last blink and the appearance of the first dry spot in the tear film. Final TBUT was calculated by averaging the three values [19].
Efficacy assessments: Reduction in IOP from baseline was considered as primary outcome of the study. IOP measurement was done with Goldman applanation tonometry and three readings one minute apart were recorded for each patient. Mean of all the three readings were taken as the final IOP of each patient. All ophthalmological examinations were performed by the same examiner at a fix time (11am±30 minutes) of the day at every visit. Percentage of patients with IOP ≤18 mmHg at least one visit during the study period, was analysed and considered as the responder rate.
Safety assessments: Safety assessments consisted of evaluation of adverse events and vital signs. Best corrected visual acuity, dilated fundoscopy, automated perimetry and gonioscopy (screening visit only) were performed. In addition, specific ocular safety i.e., conjunctival hyperemia (at baseline, week 2 and week 12) and TBUT (at baseline, week 4 and week 12) were also assessed.
Statistical Analysis
Twenty two patients in each treatment arm were sufficient to detect a difference of 1.5 mmHg in IOP between any two groups, calculated by assuming standard deviation of 3.5 mmHg. Power of test was prefixed to 80% and level of significance was taken as 0.05. This sample size was also sufficient to detect a 1.5 mmHg change in IOP from baseline by using paired t-test.
Last Observation Carried Forward (LOCF) method was used for lost to follow-up data. LOCF was considered when patient received at least one dose of study medication, and had at least one on therapy efficacy assessment. All statistical analyses were performed using SPSS software version 20.0.
Results
Patients disposition: Forty six patients were selected according to the criteria and were randomised. Patients who received at least one dose of medication were considered as safety population. Forty four patients were completely studied without any major protocol violation and were considered as per protocol population. Last Observation Carried Forward (LOCF) method was used for lost to follow-up data.
Demographic characteristics: Male predominance was seen in both group (56.5% male vs 43.5% female, in both group). But this difference in gender distribution was statistically insignificant. The Mean age of the patients for both groups was different (43.61±11.38 years vs. 45.60±12.37 years). But this difference in age was also statistically insignificant [Table/Fig-1].
Demographic characteristics and basic ophthalmologic parameters of newly diagnosed POAG patients.
| Category | Group 1 | Group 2 | Total |
|---|
| SexMaleFemale | 13(56.5%)10(43.5%) | 13(56.5%)10 (43.5%) | 26(56.5%)20(43.4%) |
| Mean age (years)SDRange | 4210.5925 to 66 | 43.6611.2925 to 65 | 43.6111.3823 to 70 |
Efficacy assessment: Intraocular pressure: Mean and SD of baseline IOP for treatment of Group 1 and 2 was 26.25±2.69 and 25.36±1.93 mmHg respectively. Importantly there was no statistically significant difference in baseline IOP between the treatment groups (p=0.324). There was sustained decrease in IOP, from baseline to final visit (i.e.3 month) (26.25±2.69 mmHg versus 16.97±1.88 mmHg; p<0.0001 for Group 1 and 25.36±1.93 mmHg versus 17.26±1.83 mmHg; p<0.0001 for Group 2) [Table/Fig-2].
Mean (SD) of IOP (in mmHg) of treatment groups at follow-up visit.
| Baseline | Week 2 | Week 4 | Week 6 | Week 12 |
|---|
| Group 1 | 26.25 (2.69) | 18.00 (1.94) | 17.42 (1.93) | 17.07 (1.91) | 16.97 (1.88) |
| Group 2 | 25.36 (1.93) | 18.32 (1.94) | 17.7 (1.89) | 17.46 (1.93) | 17.26 (1.83) |
We also observed the responder rate in terms of number of patients achieved IOP <18 mmHg at 12 week and found that it was comparable for each treatment groups. At the end of our study both the BAK free and BAK containing formulations of Latanoprost produced similar IOP response profiles. It was 54.54% for both Group 1 and Group 2.
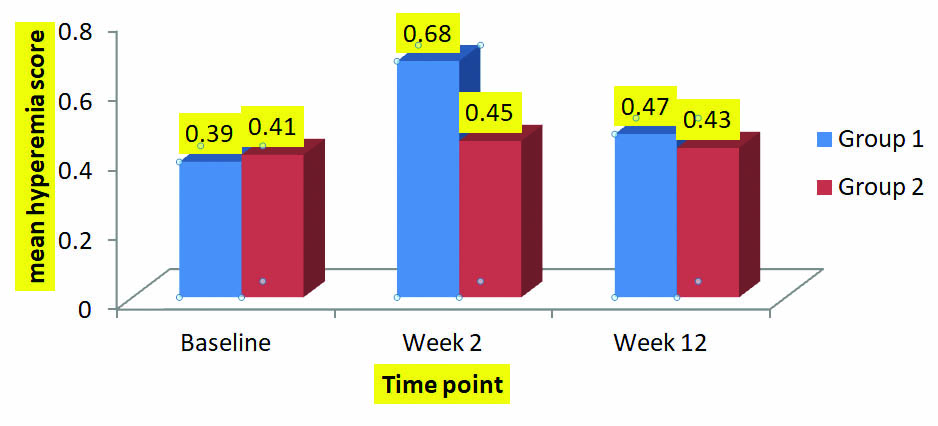
Safety assessment: Hyperemia scores were compared between groups at week 2. It was found that there was difference in scores between the groups at week 2. Difference was significant, Group 1 vs. Group 2 (p=0.025) at week 2. The differences were become insignificant at week 12 between groups (p=0.73 for group 1 vs 2) as well as from baseline value [Table/Fig-3].
Mean Hyperemia scores of Group 1and Group 2 at different follow-up visit.
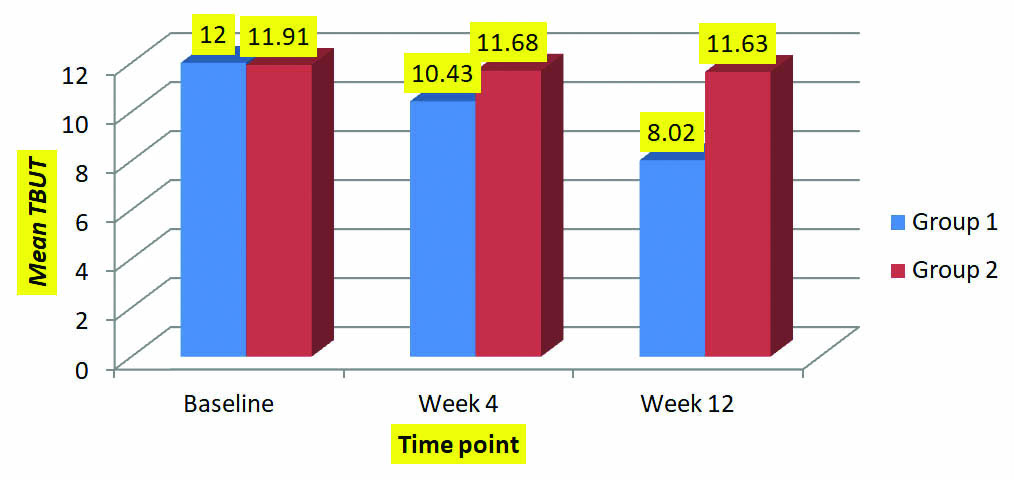
Tear Break-Up Time (TBUT): Tear break up time (TBUT): Statistically similar (p=0.91) Mean TBUT was observed for both group at baseline (12.00±2.22 and11.91±2.10 seconds). It was noted that mean TBUT for group 1 was reduced significantly at week 4 (p<0.0001) and week 12 (p<0.0001). Reduction in mean TBUT was also observed for group 2 at week 4 and week 12 but it was insignificant.
When mean TBUT values were compared between two groups at different point of time (week 4 and week 12) then there was significant difference in mean TBUT at week 4 (p<0.043) and week 12 (p<0.0001) [Table/Fig-4].
Mean TBUT of two groups at different follow-up visits.
Discussion
This study was performed to disclose various pros and cons regarding efficacy and ocular safety of BAK preserved latanoprost (0.005%) and preservative free latanoprost (0.005%) ocular solution on POAG. In our prospective randomised study, we found that both preparation produced similar IOP reduction throughout the study and was met equivalence criterion. Maximum IOP lowering efficacy achieved after two weeks of treatment and maintained through three months for both preparations.
The results of this study are consistent with randomised, investigator masked study of Sanford M which demonstrated that preservative free latanoprost was non-inferior to BAK preserved latanoprost in terms of IOP control. Furthermore a systematic literature review by Cucherat and colleagues demonstrated that there was no statistically significant difference in IOP at 3 months was seen between two formulations. A prospective, international, multicentre randomised investigator masked parallel group trial by Rauland JF et al., showed mean IOP reduction was -8.6±2.6 mmHg on preservative free latanoprost and -9±2.4 mmHg on BAK containing latanoprost, confirming non-inferiority of preservative free latanoprost to BAK containing latanoprost [20-22].
Additionally increased hyperemia score at week 2 for BAK preserved latanoprost well correlated with previous reports that BAK has been shown to worsen conjunctival inflammation [23,24]. A prospective study performed on 40 newly diagnosed POAG patients by Tomic M et al., and found that, eight out of 40 patients complained about mild to moderate hyperemia within the week after starting the BAK containing IOP lowering topical agent. But at the end of the study (at 3 month) only three patients had similar complaint of hyperemia [25]. Contradictory to previous findings, Schwartz GF et al., reported the results of the retrospective analysis of three large prescription databases suggesting that open angle glaucoma and ocular hypotensive patients newly treated with BAK preserved latanoprost were not significantly likely to develop dry eye, ocular infection, or ocular surface disease as evidenced by additional coding for these disorders during the first year of treatment [26].
TBUT evaluations were done for each group at baseline, week 4 and at week 12. Result was in accordance with other studies. Ammar DA et al., found that BAK has significant in vitro cytotoxicity to cultured ocular epithelial cells. In this study it was found that cytotoxicity of different topical medication was in accordance with the concentration of BAK instead of type of active medication. Toxicities were comparable for different PGs analogues with similar concentration of BAK [27]. A study by Crichton et al., reported tear film instability in POAG patients at 12 weeks of treatment with different types of PGs, containing preservative other than BAK. But the difference in TBUT at 12 week of treatment was not significantly different from base line values. TBUT {bimatoprost: 9.7 s (6.1), travoprost: 9.5 s (5.8), latanoprost: 9.8 s (5.0)} among subjects at latanoprost-treated baseline (p≥0.664). At week 12, there were no significant differences in TBUT {bimatoprost: 9.7 s (5.7), travoprost 9.7 s (5.0), latanoprost: 9.3 s (4.0)} among the treatment groups (P≥0.379) [18]. In the study of Walimbe T et al., it was found that mean TBUT increased significantly from baseline (3.67±1.60 seconds) to 5.03±2.64 and 6.06±3.39 seconds after 28 and 56 days of treatment with BAK free latanoprost respectively (p<0.0001) [2]. Horsley MB et al., observed 40 eyes of 20 patients using latanoprost with BAK was switched to travoprost with sofZia. Mean TBUT prior to starting travoprost was 2.02±0.71 seconds and increased to 6.34±1.31 seconds 8 weeks after the switch (p<0.001) [28].
Limitation
The current study did not include other prostaglandin analogues (e.g., travoprost, tafluprost or bimatoprost) as comparators. A limitation of the current study is that latanoprost formulations preserved with sofZia or polyquaternium1, or with other marketed prostaglandin analogues preserved with sofZia or polyquaternium1 were not compared. Another limitation of our study, we did not compared the effective preservation of BAK vs. BAK free preparations.
Conclusion
This study concluded that BAK preserved latanoprost as well as preservative free latanoprost ophthalmic solutions are equally effective in lowering of IOP in POAG patients. But it was also observed that chronic use of BAK containing latanoprost may deleteriously affect the ocular surface especially in sensitive patients or patients having a compromised ocular surface. Therefore, tolerability and efficacy should not only be considered while prescribing ophthalmic solution especially in ophthalmic diseases where long term medication required. This consideration becomes more important in subgroup of patients who already have compromised ocular surface like dry eye etc.