CVT refers to thrombus formation within the venous sinuses draining the brain. It is an uncommon form of cerebrovascular disease and represents almost 0.5% to 3% of all stroke types [1]. Early diagnosis of this entity is important, as prompt institution of anticoagulation can reverse the pathology and significantly reduce mortality and long term sequelae [2]. The aetiology which varies widely may also depend on the racial differences among the population and the geography of an area [3]. A systematic study in this aspect is lacking from Kerala. Hence, this study was conducted focussing on the clinical presentation, imaging features and aetiology of CVT from a tertiary care teaching institution in northern Kerala.
Materials and Methods
This was a retrospective, observational, non-interventional study conducted in a tertiary care referral hospital in Southern India. Patients were included in the study only if CVT was confirmed with MR Venography (MRV). Between the period of June 2015 and June 2016 a total of 35 patients met the inclusion criteria. In some cases, Computed Tomography (CT) scan was also taken prior to confirmation with MRV. A detailed history was taken from the patients and/or the bystanders, with focus on identifying the risk factors. The duration of symptoms, the location and type of imaging abnormalities were noted. A structured neurological examination was done in all patients.
All patients were investigated with complete blood count, erythrocytic sedimentation rate, blood sugars, renal and liver function tests, prothrombin time and International Normalised Ratio (INR), activated Partial Thromboplastin Time (aPTT) and sickling test. Antinuclear Antibody (ANA) and Antiphospholipid Antibodies were done in 24 patients. The rest 11 had some other definite aetiology established. Thrombophilia work up was planned in patients in whom no obvious aetiological risk factor could be identified. Out of 13 such patients, serum homocysteine, protein C, protein S and antithrombin III deficiency was done only in six patients due to resource limitation.
All patients were treated with either unfractionated heparin 800 units/h infusion to target an aPTT of 2-2.5 times the control, or subcutaneous low molecular weight heparin 40 mg twice daily. Oral anticoagulation was initiated with warfarin after five-days and was titrated to maintain an INR between 2-3.
Results
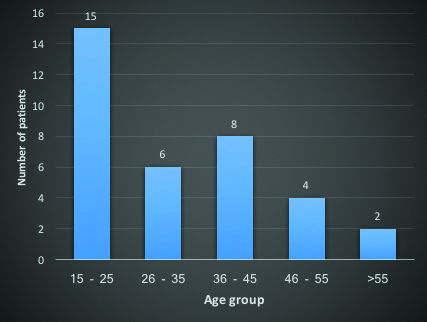
During the study period, a total of 35 cases were admitted with a diagnosis of CVT. The mean age of presentation was 32.7±15.5 years. The youngest person was 15 years and the eldest person was 88 years of age [Table/Fig-1]. Out of the 35 patients, 21 (60%) were females and 14 (40%) were males.
Age group distribution of patients.
Headache was the most common presenting symptom seen in 28 patients (80%). Among them, 10 patients (35.7%) had thunderclap headache. Rest 18 patients (64.3%) had insidious onset of headache. The next most frequent symptom was vomiting, which was seen in 15 patients (42.9%). Seizure was present in 12 patients (34.3%). The semiology was generalised tonic clonic type in all the patients. Visual abnormality was present in 11 (31.43%) patients, of which three had double vision and eight had blurring of vision. Altered sensorium was present in nine patients (25.7%). Limb weakness was present in seven patients (20%). Fever, not attributable to any infection, was present in five patients (14.3%). Other symptoms noted in our study were photophobia in two patients (5.7%), one patient (2.9%) each with slurring of speech, memory impairment, abnormal behaviour, tinnitus and pain in the eye [Table/Fig-2].
Symptoms in patients with CVT.
| Symptoms | Number of patients (%) |
|---|
| Headache | 28 (80%) |
| Vomiting | 15 (42.9%) |
| Visual abnormality | 11 (31.4%) |
| Seizures | 12 (34.3%) |
| Altered sensorium | 9 (25.7%) |
| Limb weakness | 7 (20%) |
| Fever | 5 (14.3%) |
Focal neurological deficits were seen in 10 patients (28.6%). Five patients (14.3%) had hemiparesis. Two patients (5.7%) each had brachial monoparesis, bilateral lateral rectus palsy and Broca’s aphasia. One patient (2.9%) had unilateral upper motor neuron type facial nerve palsy.
Fundus examination showed papilloedema in 11 patients (31.4%). Lumbar Puncture (LP) was performed in 8 of the 35 patients. Rest had one or more contraindication for the procedure. Mean opening CSF pressure was 266.3±72.7 mm of CSF (range 180 to 390 mm of CSF).
Imaging with CT head was performed in 27 out of 35 patients. It was normal in three patients (11.1%) and 24 patients (88.9%) had some abnormality. The most common abnormality was intraparenchymal haemorrhage seen in 7 patients (25.9%), followed by haemorrhagic infarct in 6 patients (22.2%). Five patients (18.5%) showed hyperdense venous sinuses and 4 patients (11.1%) had pure infarct. “Empty triangle sign” was seen in two (7.4%) patients. Two patients had cortical Subarachnoid Haemorrhage (SAH) and one patient (3.7%) had subdural haemorrhage.
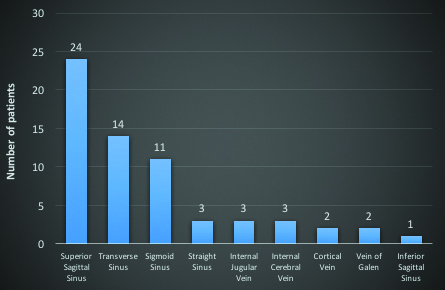
MRV was done in all patients. The most common sinus involved was Superior Sagittal Sinus (SSS), in 24 patients (68.6%), followed by transverse sinus in 14 patients (40%). Sigmoid sinus was involved in 11 patients (31.4%). Three patients (8.6%) each had involvement of straight sinus, internal cerebral vein and internal jugular vein. Cortical veins were involved in two patients (5.7%). Vein of Galen was thrombosed in two patients and one patient (2.9%) had thrombosis of inferior sagittal sinus [Table/Fig-3].
Pattern of thrombosed sinus in magnetic resonance venography.
A definite aetiology for CVT was identified in 22 patients (62.9%). The most common aetiology was secondary polycythemia, seen in four patients (11.4%). Three cases (8.6%) were postpartum CVT. Three patients had CVT secondary to injection of L-Asparaginase. Other aetiologies found out were, meningitis in two patients (5.7%), one patient (2.9%) each with polycythemia vera, hyperhomocysteinemia, protein C deficiency, antithrombin III deficiency, factor V Leiden mutation, systemic lupus erythematosus, mixed connective tissue disorder, Behcet’s syndrome, acute promyelocytic leukaemia, sickle cell disease, immune thrombocytopenic purpura and intravenous immunoglobulin treatment, oral contraceptive use and dehydration [Table/Fig-4] [4].
| Aetiology | Number of Patients (%) |
|---|
| Polycythemia | 5 (14.3%) |
| Drug Induced | 5 (14.3%) |
| Hereditary thrombophilia | 4 (11.4%) |
| Connective Tissue Disorder | 3 (8.6%) |
| Postpartum | 3 (8.6%) |
| Hematological Dyscrasia | 3 (8.6%) |
| Meningitis | 2 (5.7%) |
| Dehydration | 1 (2.3%) |
| Unknown | 13 (37.1%) |
Discussion
To date, this is the largest study of CVT from Kerala. The mean age in our study was 32.6 years. In comparison, in the largest international study of CVT, the international study of cerebral venous thrombosis (ISCVT), the mean age group was 39.1 years [5]. In a large Indian study by Narayan D et al., {from the Nizam’s Institute Venous Stroke Registry (NIVSR)}, the mean age was 31.3 years [6]. Most number of the patients in our study were in the 15-25 year age group (42.9%), conforming with previous studies that CVT occurs mostly in young patients.
There was a female preponderance in our study, with a male to female ratio of 2:3. This was also observed in ISCVT, where the male to female ratio was 1:3 [5]. The higher incidence in females is due to gender specific risk factor like pregnancy, puerperium, use of oral contraceptives and higher incidence of connective tissue disorders in females, which is a major risk factor for CVT [7]. However, this trend was not observed in recent studies from India, where a male preponderance was observed [6,8].
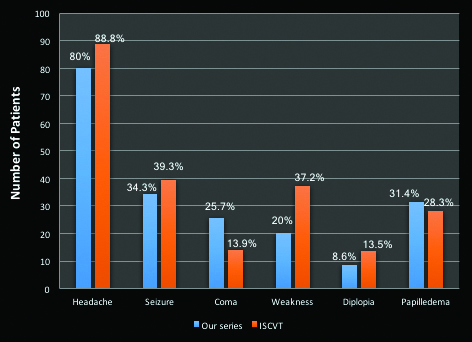
Headache was the most common presenting symptom seen in 28 (80%) patients. In the ISCVT 89.9% patients had headache [4] [5]. This is in contrast to arterial stroke where headache is usually absent. Headache is caused by two factors- raised intracranial pressure and the local involvement of the pain sensitive fibres in the dura mater, caused by distention of the sinus wall [9]. Importantly, headache may be the only symptom of CVT in many patients. In our study 12 patients (34.3%) had headache as the only symptom of CVT. In a study by Cumurciuc R et al., which analysed this aspect of CVT, 28 out of 123 patients (23%) had only headache as the sole manifestation of CVT [10]. In most patients the headache is usually insidious in onset. However, thunderclap headache can be observed in a few patients with CVT. In our study 10 patients (28.6%) had thunderclap headache, an incidence higher than reported in previous studies [10,11]. In such patients, SAH needs to be excluded by appropriate imaging or LP. In our study, 7 patients (20%) had no headache. Coutinho JM et al., analysed data from ISCVT to characterise patients without headache [12]. They found out that such patients were significantly older, less often females and had shorter interval from onset of symptoms to admission. Paresis and seizures were more common in these patients and papilloedema was less seen. Isolated cortical vein thrombosis was more common and multiple sinus involvement was less common in such patients. In contrast, we observed that in our CVT patients without headache, there were almost equal number of males and females. It included patients in all age group as well. Conforming to the previous study, papilloedema was not observed in any of these patients.
Seizures were seen in 12 patients (34.3%) in our study. This is almost similar to incidence found in ISCVT (49.6%), and NISVR (45.2%). The semiology was generalized tonic clonic type in all our patients. In a study by Breteau G et al., 10% had focal and 37% had generalized seizures [13]. We also observed that, all but one patient with seizures had thrombosis of SSS and most had intraparenchymal bleeding on CT head. Involvement of SSS and cortical location of the insult increases the risk of seizures in CVT.
In the present study, 9 patients (25.7%) presented with altered sensorium. To avoid the bias of postictal confusion, reassessment was made at least one day later in patients with seizures. In comparison, data from the National Mexican Registry of Cerebral Vascular Disease (RENAMEVASC) study showed that altered mental status was seen in 47.5% of the cases [14]. In a study by Banakar BF et al., from a tertiary care centre in Northern India, 54% had altered sensorium [15]. In multiple studies, coma at presentation was an independent factor adversely affecting the long term prognosis of the patients [5,15,16].
Visual abnormality was present in 11 patients (31.43%). Three patients had horizontal diplopia and 8 patients had blurring of vision. All patients with visual abnormality had papilloedema on fundus examination. Two patients with diplopia had bilateral lateral rectus palsy due to raised intracranial pressure.
Focal neurological deficits were seen in 10 patients (28.6%). Hemiparesis was the most common deficit seen in 5 patients, followed by monoparesis, aphasia, bilateral lateral rectus palsy and facial nerve palsy. Incidence of focal deficits varied widely in studies. In the ISCVT 49.5% patients had focal signs [5]. In Indian studies, the NISVR had 27.6% with stroke like presentation, whereas it was 47.7% in a study by Pai N et al., and 38.1% in another study by Parikh PM et al., [8,17]. In conformity with our study, hemiparesis was the most frequent deficit seen in all these studies.
Fever was present in six patients, out of which one had tuberculous meningitis. Rest five patients (14.3%) did not have any evident focus of infection. CSF, blood and urine cultures were sterile in these patients and none had otitis media. Such non-infectious cause of fever may be neurogenic in origin. Neurogenic fever is well described in patients with traumatic brain injury, SAH and brain tumours [18]. Injury to the hypothalamus is the postulated mechanism of neurogenic fever [19]. Except in cases of infective cavernous sinus thrombosis, fever has never been previously described in CVT, to the best of our knowledge.
Papilloedema was an important fundoscopic finding seen in 11 patients (31.4%). In the ISCVT the incidence of papilloedema was 28.3% [5]. It was more observed in patients with involvement of transverse sinus or SSS in our study. Idiopathic intracranial hypertension (IIH) is a headache syndrome characterized by chronic headache, papilloedema and increased CSF pressure in LP study without any mass lesion in the brain. CVT is a well-established cause for IIH [20]. In our study, three patients were initially admitted with a diagnosis of IIH. Only on subsequent evaluation with MRV, CVT was identified. This highlights the importance of considering CVT as a cause for IIH. A summary of comparison of the clinical profile of our study with ISCVT cohort is given in the [Table/Fig-5].
Comparison of clinical profile of our study with ISCVT cohort.
LP was performed in eight of the 35 patients in our study. This is not a mandatory investigation to diagnose CVT, but is important to exclude secondary causes like meningitis and to diagnose IIH in suspicious cases. The mean CSF pressure was 266.3±72.7 mm of CSF (normal range 50-150 mm of CSF). In all cases in which LP was performed CSF pressure was raised. Hence, it is prudent to treat cases of CVT with measures to lower intracranial pressure like mannitol, acetazolamide or frusemide in selected cases depending upon clinical judgment.
Imaging with CT head was done in 27 patients out of which it was normal only in three cases (11.1%). In a study of 11 patients with CVT by Rao KC et al., normal CT was found in four patients (36.4%) [21]. Specific signs of CVT in CT include the cord sign and the empty triangle sign [22,23]. This was observed in five and two patients respectively in our study. CT alone could accurately diagnose CVT in seven patients (25.92%) implicating the importance of this imaging technique.
We observed that the most common sinus involved was SSS in 24 patients (68.6%), followed by transverse sinus in 14 patients (40%). In the ISCVT, transverse sinus was the most common sinus involved [5]. This may be due to more number of cases secondary to mastoiditis and ear infection, which was less in our study.
Aetiology varied widely in our study. Secondary polycythemia was the most common aetiology seen in four patients (11.4%), a much higher incidence than seen in NISVR (1.8%) and ISCVT (2.8%). In contrast, CVT due to pregnancy and puerperium was seen in only three patients (8.6%), whereas the ISCVT published in 2004, had 20.1% obstetric cases. This may be due to improved antenatal and obstetric care over the years. Drugs which have caused CVT in our study include oral contraceptives, L-Asparaginase given for treatment of acute leukaemia and intravenous immunoglobulin. Hereditary thrombophilia was present in four patients (11.4%). In the largest study from India, 18% patients with CVT had one or more thrombophilia markers [8]. One case of CVT occurred in a patient with acute promyelocytic leukaemia. CVT is a rare manifestation of leukaemia and underlies the importance of searching for malignancy in all cases of CVT [24]. Sickle cell disease is a disorder prevalent among our population studied, and is a known risk factor for CVT [25,26]. We found one case of CVT secondary to sickle cell disease. It is our practice to do a sickling test and high performance liquid chromatography in all patients with CVT to exclude this potentially treatable disorder. The aetiology could not be found out in 13 patients (37.1%) in our study. In comparison aetiology was unknown in 12.5% cases in the ISCVT and in 66.7% in the RENAMEVASC study] [5,14]. Serial follow up of patients is required to establish an aetiology in many cases as the underlying disorder may not be fully manifested during the first presentation.
Limitation
Our study had certain limitations. Since CVT can be rapidly fatal in some cases, Magnetic Resonance Imaging (MRI) of the brain would not be taken and hence such cases would be missed. This can lead to underestimation of fatal cases. In the other end, cases of CVT with mild headache alone would not be investigated with MRI brain and such cases would also be missed. Estimation of protein C, protein S and antithrombin III were done during the acute phase of the thrombus and can lead to erroneous values in some cases. The study was not designed to follow up the patients or to assess mortality predictors.
Conclusion
CVT presents with a wide variety of symptoms and hence a high index of suspicion is required for the diagnosis. CVT should be considered in the differential diagnosis of thunderclap headache. All cases of IIH should be investigated for a primary CVT. An important novel feature in some of our patients was neurogenic fever. CT can accurately diagnose CVT in a significant number of cases. SSS was the most common sinus involved in our study. A definite aetiology may not be identified in a significant number of cases and hence serial follow up is necessary.