Role of Salivary Electrolytes in Prevalence of Dental Caries among Diabetic and Non-Diabetic Adults
UK Ambikathanaya1, Usha Hegde2, HM Tippeswamy3, Mohamad Ayas4
1 Lecturer, Department of Conservative Dentistry and Endodontics, JSS Dental College and Hospital, Mysore, Karnataka, India.
2 Professor, Department of Oral Pathology, JSS Dental College and Hospital, Mysore, Karnataka, India.
3 Reader, Department of Public Dentisty, JSS Dental College and Hospital, Mysore, Karnataka, India.
4 PG Student, Department of Oral Pathology, JSS Dental College and Hospital, Mysore, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. UK Ambikathanaya, Lecturer, Department of Conservative Dentistry and Endodontics, JSS Dental College and Hospital, Mysore-570015, Karnataka, India.
E-mail: ambikathanayauk@gmail.com
Introduction
Diabetes mellitus has been linked with an increased risk of caries, gingivitis and periodontal disease. Dental caries is more prevalent and even more severe in diabetic patients than non-diabetics. The aetiology and pathogenesis of dental caries are known to be multifactorial. The secretion rates and quality of saliva are important not only in caries development but also for remineralisation. As there is an alteration of the salivary constituents in diabetic patients its constituents has gained much importance as a diagnostic and therapeutic tool. Various factors that regulate the caries activity balance are the pH of saliva and concentration of various ions including free calcium, phosphate, sodium, chloride, potassium and fluoride ions in the saliva.
Aim
To investigate the association of salivary electrolyte concentration with dental caries among diabetic and non-diabetic individuals, of different age groups.
Materials and Methods
Ninety-six Patients were evaluated for the study in which forty eight were considered for control and forty eight for diabetic, in which these control group and diabetic group were further subdivided into young adults (20-39 yrs) and old age adults (40-64 yrs) with twenty four patients each. Young adult age group and adult age group had sub groups with caries and without caries group having twelve patients each respectively. Saliva was collected from the individuals, centrifuged and the supernatant obtained was assessed for the salivary electrolytes: sodium, potassium, calcium, phosphorous and chloride levels using an Erba autoanalyser. The results obtained were tabulated, statistically analysed using Independent sample t-test and conclusions drawn.
Results
The salivary electrolytes– sodium, potassium, calcium, and phosphorous showed a significant decrease in caries active diabetic patients of young adult group. But in the older adult group K and Cl levels were statistically higher in caries active patients. Potassium & Phosphorous were statistically higher in young adult age group (Diabetic and Non diabetic) without caries and Potassium only in adult age group.
Conclusion
Salivary electrolyte plays a significant role in prevalence of dental caries in young diabetic individuals when compared to non-diabetic individuals and old age adults with and without diabetics. Therefore, maintaining the salivary electrolyte concentration in young diabetic individuals helps in remineralisation of the tooth which prevents decay.
Oral fluids, Remineralisation, Systemic diseases
Introduction
Diabetes mellitus is a clinical syndrome characterised by hyperglycaemia due to deficiency/diminished effectiveness of insulin [1]. Type 1 diabetes is due to destruction of insulin producing pancreatic β cells due to a T cell mediated autoimmune process. Type II diabetes develops due to a combination of insulin resistance and impaired insulin secretion [2]. Diabetes mellitus condition provides a suitable environment for the progression of dental caries compare to non-diabetic. Patients with diabetes are more prone for periodontal diseases, salivary dysfunction, oral mucosal diseases and oral infections [1].
Saliva plays an important role in maintaining the oral health of the patients. It has defensive potential comprising both immunologic and enzymatic mechanism. It had been documented that many constituents of serum and saliva, both organic and inorganic have potential protective role, which includes calcium, phosphate and fluoride ions. The levels of these ions in saliva acts as a diagnostic and therapeutic tool in various local and systemic conditions which has established the importance of assessment of salivary constituents [1].
As a diagnostic fluid, saliva offers distinctive advantages over serum because it can be collected non-invasively by individuals and even by patients. It doesn’t require any special equipment for collection and storage. Unlike blood, saliva doesn’t clot and has the advantage for person in whom blood drawing is difficult as in obese and haemophilic patients [3]. A study revealed a large number of functions, mediated by both the organic and inorganic components of saliva that should be considered in assessments of the effects of human saliva on dental caries [4].
A study has shown that diabetes mellitus patients have presented a decrease in salivary flow and pH [1]. A study was carried out on salivary pH and dental caries in diabetes mellitus which states that diabetes mellitus have a direct effect on salivary pH reducing it from normal levels irrespective of diet [5]. Reduced salivary flow rates in diabetic patients is due to diabetes induced neuropathic changes in salivary parenchyma with lymphocytic gland infiltrate similar to the one occurring in the pancreas of these diabetic patients [6].
In young patients the secretion rate of saliva is more and less viscous when compared to old age adults. Healthy older individuals have altered acinar structure and mildly reduced salivary production. Accordingly decreased salivary secretions in old age patients is due to normal physiological process that takes place in salivary glands as age advances and the adverse effects of anticholinergic drugs on salivary secretions are the aetiological factors for salivary dysfunction in old age adults [7].
Diabetes mellitus is one such condition where in the salivary constituents are changed, but its impact on caries is not clearly explained [8].
Therefore, the purpose of this study was to evaluate the salivary electrolyte concentration-sodium (Na), potassium (K), chloride (Cl), calcium (Ca), phosphorous (P) among young adults (20-39 years) and older adults (40-64 years) with and without caries in control (non-diabetic) and test (diabetic) groups.
Materials and Methods
A case control study was carried out between two groups’ diabetic and non-diabetic. Each group based on the age factor subdivided into young adults and old age adults with and without dental caries. The study had been carried out for one year and two months, (July 2015 to August 2016) respectively.
Patients between age group of 20-39 years (young adult group) and 40-64 years (older adult group) who came for dental treatment in the Outpatient Department of Conservative Dentistry and Endodontics were considered for salivary evaluation. By obtaining their consent, their diabetic status was assessed by routine random blood and urine sugar levels by Erba auto analyser and Benedict’s method respectively. Patients with random blood sugar greater than 160 mg/dl and urine sugar of 0.5% (green colour) were considered as diabetic and values below that as control.
Subjects of either sex, between 20-39 years and 40-64 years age group who were diabetic/non diabetic with OHI of 0.1-1.2 (good oral hygiene) were included in the study. Subjects of age below 20 years and above 64 years with any other oral lesions, systemic illness, tobacco habits, intake of medications causing xerostomia and OHI of 3-1.6 were excluded from the study. OHI was assessed and only subjects in both control and study group with OHI of 0.1-1.2 were taken for the study because poor oral hygiene is also one of the contributing factor for progression of caries.
The sample size was calculated based on assumption of standard deviation 0.5 and 0.75 & mean difference of 0.52 between diabetic and control group. The power used 90% and 5% of error the sample size was obtained 24 in each group. (n-mastering software Vellore)
A total 96 patient’s were included in the study. They were broadly divided into two groups- Control and Experimental /diabetic group based on their diabetic status. In each group they were divided into two age groups, 20 to 39 years (young adults) and 40 to 64 years (older adult), since older individuals show decreased salivary secretion as a part of normal ageing process. They were further subdivided into patients with caries and without caries [Table/Fig-1].
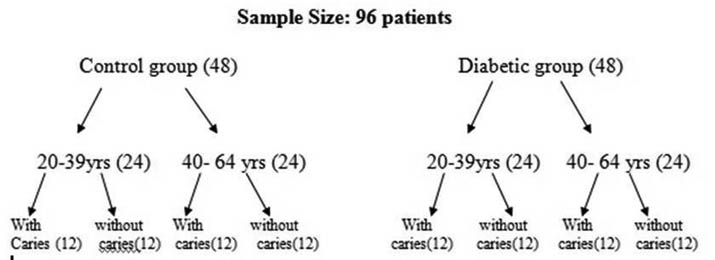
Study Design
Ethical clearance was taken and informed written consent was obtained from each of the subjects and dental caries was assessed using Decayed, Missing, Filled Tooth (DMFT) index.
Collection of Saliva for Salivary Analysis: The subjects were asked to rinse their mouth with 15ml of distilled water to wash out the exfoliated cells. A 5ml of unstimulated whole saliva was then collected by asking the subjects to expectorate into sterile tubes. The collected saliva was centrifuged and the supernatant obtained was evaluated using an Erba autoanalyser Chem 5X N110825 for Na, K, Cl, Ca, P. When the evaluation could not be done immediately, it was stored at 40C for subsequent analysis.
Statistical Analysis
Independent sample t-test was used to correlate the salivary electrolytes concentration among the groups. A p-value of 0.05 or less was considered significant. Results are presented as mean±standard deviation. SPSS 17 software analysis was used.
Results
In the control group there was no significant change in the salivary electrolyte levels considered, between persons with or without caries in young adult age group [Table/Fig-2a]. But in the older adult group K and Cl levels were statistically higher in caries active patients [Table/Fig-2b].
Control group with and without caries in 20 to 39 years young adult age group (A) N=12
| Electrolytes | A Groups | MeanMg/dL | Std deviation | t-value | p-value |
|---|
| Sodium | Non-diabetic with cariesNon-diabetic without caries | (197.7)(166.6) | (70.0)(46.5) | 1.28 | 0.214 |
| Potassium | Non-diabetic with cariesNon-diabetic without caries | (10.24)(8.93) | (5.22)(4.37) | 0.663 | 0.514 |
| Chloride | Non-diabetic with cariesNon-diabetic without caries | (17.80)(17.54 | (10.7)(9.24) | 0.062 | 0.951 |
| Calcium | Non-diabetic with cariesNon-diabetic without caries | (7.67)(9.29) | (4.13)(3.39) | -1.049 | 0.305 |
| Phosphorous | Non-diabetic with cariesNon-diabetic without caries | (5.99)(8.62) | (3.64)(5.34) | -1.408 | 0.173 |
Control group with and without caries in 40 to 64-year-old adult age group (B). N=12.
| Electrolytes | B Groups | Mean | Std deviation | t-value | p-value |
|---|
| Sodium | Non-diabetic with cariesNon-diabetic without caries | (187.2)(142.0) | (86.94)(78.3) | 1.338 | 0.195 |
| Potassium | Non-diabetic with cariesNon-diabetic without caries | (14.5)(10.0) | (2.622)(5.30) | 2.604 | 0.016 |
| Chloride | Non-diabetic with cariesNon-diabetic without caries | (34.9)(21.2) | (17.78)(10.41) | 2.299 | 0.031 |
| Calcium | Non-diabetic with cariesNon-diabetic without caries | (6.97)(7.72) | (3.72)(3.10) | -0.535 | 0.598 |
| Phosphorous | Non-diabetic with cariesNon-diabetic without caries | (9.48)(12.3) | (3.93)(6.23) | -1.366 | 0.186 |
In the diabetic group, except for the Cl levels all the other four electrolytes showed a significant decrease in caries active patients in the young adults [Table/Fig-3a] and no changes of significance was noted in the older adults [Table/Fig-3b].
Diabetic group with and without caries in 20 to 39 years young adult age group(A) N = 12.
| Electrolytes | A Groups | MeanMg/dL | Std deviation | t-value | p-value |
|---|
| Sodium | Diabetic with cariesDiabetic without caries | (132.1)(175.0) | (57.29)(15.66) | -2.498 | 0.020 |
| Potassium | Diabetic with cariesDiabetic without caries | (5.500)(14.78) | (3.40)(5.97) | -4.674 | 0.00 |
| Chloride | Diabetic with cariesDiabetic without caries | (25.00)(23.80) | (7.07)(16.49) | 0.231 | 0.820 |
| Calcium | Diabetic with cariesDiabetic without caries | (4.166)(10.21) | (1.21)(3.32 | -5.919 | 0.00 |
| Phosphorous | Diabetic with cariesDiabetic without caries | (12.63)(18.75) | (3.52)(1.41) | -5.587 | 0.00 |
Diabetic group with and without caries in 40 to 64-year-old adult age group(B). N = 12
| Electrolytes | B Groups | Mean | Std deviation | t-value | p-value |
|---|
| Sodium | Diabetic with cariesDiabetic without caries | (168.1)(159.4) | (52.62)(53.80) | 0.399 | 0.694 |
| Potassium | Diabetic with cariesDiabetic without caries | (13.7)(19.3) | (5.02)(10.31) | -1.671 | 0.109 |
| Chloride | Diabetic with cariesDiabetic without caries | (26.0)(24.5) | (10.44)(10.96) | 0.361 | 0.722 |
| Calcium | Diabetic with cariesDiabetic without caries | (6.70)(6.64) | (2.867)(4.73) | 0.035 | 0.972 |
| Phosphorous | Diabetic with cariesDiabetic without caries | (13.45)(12.52) | (5.56)(6.05) | 0.392 | 0.699 |
Between the control and diabetic patients without caries, K and P levels were high in young adults diabetics [Table/Fig-4a] and only K was higher in diabetics of older adult group [Table/Fig-4b].
Comparison of diabetic and non-diabetic patients without caries in 20 to 39 years young adult age group (A) N=12.
| Electrolytes | A Groups | MeanMg/dL | Std deviation | t-value | p-value |
|---|
| Sodium | Diabetic without cariesNon-diabetic without caries | (175)(166.6) | (15.6(46.5 | -0.588 | 0.562 |
| Potassium | Diabetic without cariesNon-diabetic without caries | (14.7)(8.93) | (5.97(4.37 | -2.733 | 0.012 |
| Chloride | Diabetic without cariesNon-diabetic without caries | (23.80)(17.54) | (16.49(9.24 | -1.146 | 0.264 |
| Calcium | Diabetic without cariesNon-diabetic without caries | (10.21)(9.29) | (3.32(3.39 | -0.673 | 0.508 |
| Phosphorous | Diabetic without cariesNon-diabetic without caries | (18.75)(8.62) | (1.41(5.34 | -6.342 | 0.00 |
Comparison of diabetic and non-diabetic patients without caries in 40 to 64-year-old adult age group (B) N=12.
| Electrolytes | B Groups | Mean | Std deviation | T value | P value |
|---|
| Sodium | Diabetic without cariesNon-diabetic without caries | (159.4)(142) | (53.80)(78.38) | -0.636 | 0.531 |
| Potassium | Diabetic without cariesNon-diabetic without caries | (19.3)(10.05) | (10.31)(5.30) | -2.767 | 0.011 |
| Chloride | Diabetic without cariesNon-diabetic without caries | (24.50)(21.2) | (10.96)(10.41) | -0.749 | 0.462 |
| Calcium | Diabetic without cariesNon-diabetic without caries | (6.64)(7.72) | (4.73)(3.10) | -0.661 | 0.516 |
| Phosphorous | Diabetic without cariesNon-diabetic without caries | (12.52)(12.39) | (6.05)(6.23) | -0.051 | 0.960 |
The comparison of the caries patient’s salivary electrolyte levels between control and diabetic group showed a notable decrease in all except Cl levels in young adults [Table/Fig-5a], without any significant difference in the older adults [Table/Fig-5b].
Comparison of diabetic and non-diabetic patients with caries in 20to 39 years young adult age group (A) N=12.
| Electrolytes | A Groups | MeanMg/dL | Std deviation | t-value | p-value |
|---|
| Sodium | Diabetic with cariesNon-diabetic with caries | (132.16)(197.7) | (57.29)(70.0) | 2.511 | 0.020 |
| Potassium | Diabetic with cariesNon-diabetic with caries | (5.50)(10.24) | (3.40)(5.22) | 2.632 | 0.015 |
| Chloride | Diabetic with cariesNon-diabetic with caries | (25.00)(17.80) | (7.07)(10.7) | -1.934 | 0.066 |
| Calcium | Diabetic with cariesNon-diabetic with caries | (4.166)(7.67) | (1.21)(4.13) | 2.820 | 0.010 |
| Phosphorous | Diabetic with cariesNon-diabetic withcaries | (12.63)(5.99) | (3.52)(3.64) | -4.541 | 0.000 |
Comparison of diabetic and non-diabetic patients with caries in 40 to 64-year-old age groups(B) N=12.
| Electrolytes | B Groups | Mean | Std deviation | t-value | p-value |
|---|
| Sodium | Diabetic with cariesNon-diabetic with caries | (168.11)(187.20) | (52.62)(86.94) | 0.650 | 0.522 |
| Potassium | Diabetic with cariesNon-diabetic with caries | (13.7)(14.5) | (5.02)(2.62) | 0.438 | 0.666 |
| Chloride | Diabetic with cariesNon-diabetic with caries | (26.08)(34.91) | (10.44)(17.7) | 1.483 | 0.152 |
| Calcium | Diabetic with cariesNon-diabetic with caries | (6.70)(6.97) | (2.866)(3.72) | 0.203 | 0.841 |
| Phosphorous | Diabetic with cariesNon-diabetic with caries | (13.45)(9.48) | (5.56)(3.93) | -2.016 | 0.056 |
Evaluation of the impact of alterations in the salivary electrolyte levels of diabetic patients on dental caries revealed that the decrease of Na, K, Ca, and P levels in caries active individuals of young adults was significant than in the older adult group.
Discussion
In the present study, diabetic group, except for the Cl levels all the other four electrolytes showed a significant decrease in caries active patients in the young adults [Table/Fig-3a] and no changes of significance was noted in the older adults [Table/Fig-3b]. The result evaluate that diabetes plays a vital role in salivary electrolytes concentration which indirectly promotes the progression of caries in young patients compared to older individuals. A positive correlation of other salivary electrolytes such as sodium, chloride and potassium with diabetics have been reported [3].
The role of calcium and phosphorous in dental remineralisation is well known and their concentration in whole stimulated saliva reflects their level in plaque. Study has proven that Phosphorous have caries prevention effect [9]. High levels of calcium and phosphorous in whole and parotid saliva were found to be associated with a low dental caries rate [10]. Study had shown that low levels of salivary calcium and phosphate have a modest association with caries susceptibility. Calcium and Phosphorous are known to reduce enamel solubility thus, decreasing incidence of dental caries [11].
A study which was carried out on salivary electrolytes as a biomarker in caries active type II diabetes had revealed that altered salivary electrolytes had impact on prevalence of dental caries in diabetic patients when compared to non-diabetics [12]. However, this study did not consider the normal ageing process, which is known to alter the salivary gland function.
Hence, the current study evaluated the prevalence of dental caries among diabetics and non-diabetics in young adults (20-39 years) and older adults (40-64 years) with and without caries teeth by analysing the salivary electrolytes. It was found that salivary Na, K, Ca, P levels were less in caries active individuals of diabetics in the young adult group than in older adult group. But in control group (Young adult group) there was no significant difference among these groups with or without caries, but K and Cl levels showed significantly higher levels in older age group with caries. Investigator also reported higher potassium and chloride levels in saliva from caries active individuals [10]. From this we could understand that the decreased levels of salivary electrolytes have more impact on prevalence of dental caries in young adults than the older adults.
The comparison of the salivary electrolyte level between control and diabetic patients without caries revealed high K and P levels and high P levels in young adults and older adults respectively. Similar comparison in caries active individuals showed a notable decrease in all the electrolyte levels except Cl in young adults only. These results suggest that irrespective of age group non carious individuals showed alterations of K and P electrolytes. Apart from this Ca & Na levels are also being altered in caries active individuals of young adults only.
Limitation
Within the limitations of this study, age factor plays a vital role in dental caries prevalence in diabetic conditions. In this study it had showed that diabetes had its impact on salivary electrolytes alteration resulted in prevalence of dental caries in young adults. But in old age adults apart from diabetes other factors also plays an important role in dental caries prevalence.
The probable explanation for a slight increase in Cl level in diabetic group could be the hyperchloremia seen in diabetics [12] and the decrease in other electrolytes due to the decrease in salivary output [13].
In relation to the future prospective of the present study the sodium ions from the blood are transferred to the salivary glands and secreted with the bicarbonate ions [11]. The mechanism behind this reaction has not yet been extensively studied and still requires more research.
Conclusion
Diabetes played a significant role in caries progression in young diabetic adult patients when compare to old age adults. There is a marked decrease in salivary electrolyte concentration in young adults. Therefore, maintaining the salivary electrolyte concentration in young diabetic individuals helps to prevent demineralisation of the tooth.
[1]. Hegde MN, Tahiliani D, Shetty S, Devadiga D, Salivary alkaline phosphatase and calcium in caries active type II diabetes mellitus patients:An invivo studyContemp Clin Dent 2014 5(4):440-43.10.4103/0976-237X.14280525395756 [Google Scholar] [CrossRef] [PubMed]
[2]. Gowdar IM, Almuhaiz M, Diabetes and oral health – a reviewAnn Int Med Den Res 2016 2(2):02-08. [Google Scholar]
[3]. Mittal S, Bansal V, Garg S, Atreja G, Bansal S, The diagnostic role of saliva- A ReviewJ Clin Exp Dent 2011 3(4):e314-20.10.4317/jced.3.e314 [Google Scholar] [CrossRef]
[4]. Lenander-Lumikari M, Loimaranta V, Saliva and dental cariesAdv Dent Res 2000 14:40-47.10.1177/0895937400014001060111842922 [Google Scholar] [CrossRef] [PubMed]
[5]. Goyal D, Kaur H, Jawanda MK, Verma S, Parhar S, Salivary pH and dental caries in diabetes mellitusInt J Oral & Maxillofac Pathol 2012 3(4):13-16. [Google Scholar]
[6]. Lasis TJ, Fasanmade A, A salivary flow and composition in diabetic and non – diabetic subjects NigerJ Physiol Sci 2012 27:79-82.10.1111/j.1741-2358.2009.00284.x19392837 [Google Scholar] [CrossRef] [PubMed]
[7]. Gueiros LA, Soares MS, Leão JC, Impact of ageing and drug consumption on oral healthGerodontology 2009 26:297-301. [Google Scholar]
[8]. Iqbal S, Kazmi F, Asad S, Mumtaz M, Khan AA, Dental caries and diabetes mellitusPakistan Oral and Dental Journal 2011 31(1):60-63. [Google Scholar]
[9]. Khalifa MAAA, Abouelkheir HM, Khodiar SEF, Mohamed GAM, Salivary composition and dental caries among Children controlled asthmaticsEgyptian Society of Chest Diseases and Tuberculosis 2014 63:777-88.10.1016/j.ejcdt.2014.05.003 [Google Scholar] [CrossRef]
[10]. Mass E, Gadoth N, Harell D, Wolff A, Can salivary composition and high flow rate explain the low caries rate in children with Familial dysautonomia?Pediatr Dent 2002 24(6):581-86. [Google Scholar]
[11]. Singh V, Arora R, Bhayya D, Singh D, Sarvaiya B, Mehta D, Comparison of relationship between salivary electrolyte levels and dental caries in children with Down syndromeJ Nat Sc Biol Med 2015 6:144-48.10.4103/0976-9668.14911325810652 [Google Scholar] [CrossRef] [PubMed]
[12]. Hegde MN, Tahiliani D, Shetty S, Devadiga D, Salivary electrolyte as a biomarker in caries active type II diabetes-a comparative studyNUJHS 2014 4(3):85-89. [Google Scholar]
[13]. Gopinath VK, Arzreanne AR, Saliva as a diagnostic tool for assessment of dental cariesArchives of Orofacial Sciences 2006 1:57-59. [Google Scholar]