Emphysematous Pyelonephritis (EPN) is a rare, potentially fatal necrotising pyelonephritis, with rising trend in diabetic patients (95%). E.coli is the most common uropathogen whereas Candida albicans is extremely rare. Mortality in EPN, with present management techniques, is 18%. It should be suspected in diabetic patients with pyelonephritis. Splenic abscess is an uncommon association. Contrast Enhanced Computed Tomography (CECT) of abdomen is cornerstone for diagnosis. Nephrectomy is mainstay of treatment in extensive disease. Herein, we report a rare case of EPN in 58-year-old female who presented with diabetic ketoacidosis and septicaemia caused by E. coli and Candida albicans with superadded Herpes genitalis and oral candidiasis which is unique. Right nephrectomy was done. Postoperatively, she developed splenic abscess which was managed by percutaneous drainage.
Nephrectomy, Oral candidiasis, Splenic abscess
Case Report
A 58-year-old female presented in surgical emergency with pain in right lumbar region, fever and vomiting for 5 days followed by altered sensorium for one day. She also had complaint of pain and itching over the vulva.
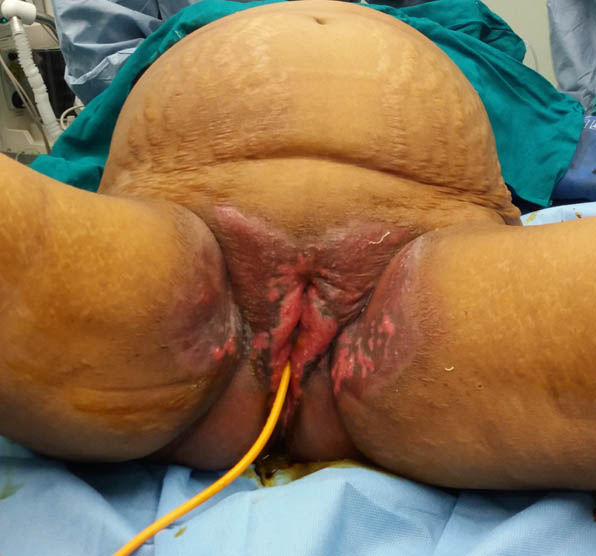
On physical examination, she was febrile, disoriented and anaemic, with pulse rate 110 per minute, blood pressure 90/60mmHg, and respiratory rate 29 per minute. The patient was average built with BMI 25.72kg/cm2 (68kg/162.6cm). Local examination revealed tenderness in right lumbar region with fullness and oedema in renal angle. Multiple polycyclic erosions on erythematous base were present over labia majora; suggestive of Herpes genitalis [Table/Fig-1]. She also had oral candidiasis.
Herpes genitalis lesion over vulva.
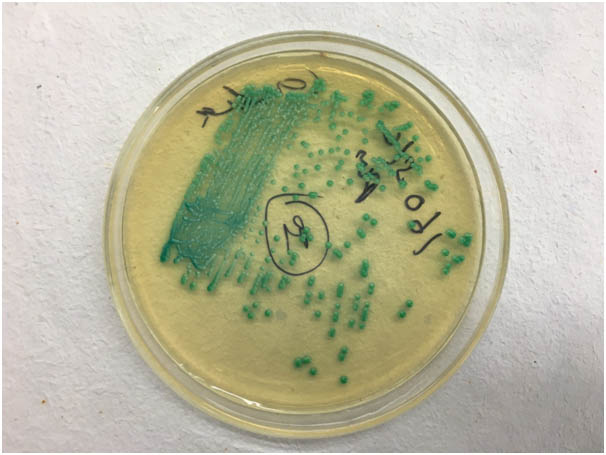
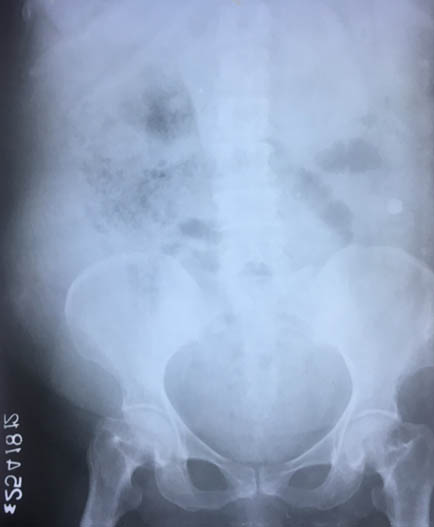
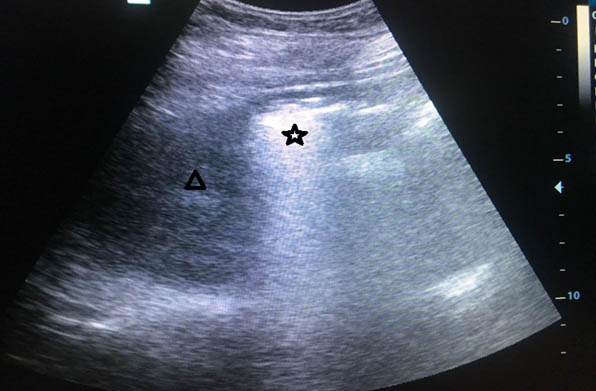
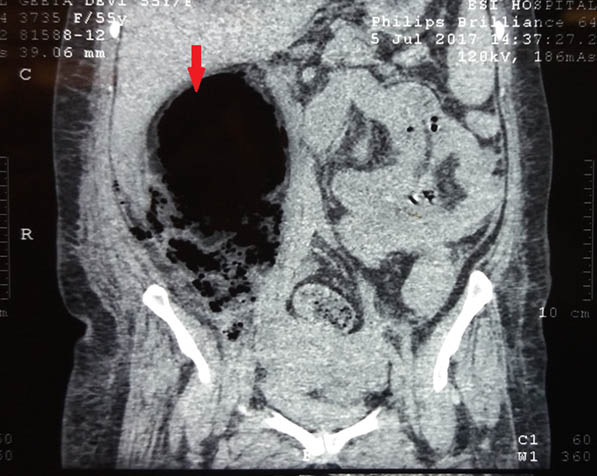
Her haemoglobin was 6.4mg/dl, TLC count 14500/mm3, serum creatinine 0.8mg/dl, random blood sugar 328mg/dl and, HBA1c 9.6%; rest of the biochemical tests were normal. ELISA for HIV was negative. Blood gas analysis revealed metabolic acidosis. Urine examination showed sugar ++ and ketones ++ with abundant pus and yeast cells on microscopy. E.coli and Candida albicans were grown on urine culture [Table/Fig-2]. X-Ray- KUB region showed ill-defined air spaces in region of right kidney [Table/Fig-3]. In ultrasound abdomen, right kidney showed posterior acoustic shadow due to air foci in region of mid and lower pole (star) and distorted right upper pole tissue (triangle) [Table/Fig-4]. CECT abdomen showed severe destruction of right kidney and replaced by a large air fluid level, with extension to right paracolic gutter, picture highly suggestive of EPN, class 3b [Table/Fig-5]. Left kidney was normal.
Candida chrome agar showing growth of C.albicans.
X-Ray KUB Region showed ill-defined air spaces in region of right kidney.
USG Abdomen: Right Kidney showed posterior acoustic shadow due to air foci in region of mid and lower pole (star) and distorted right upper pole tissue (triangle).
CECT abdomen showed severe destruction of right kidney and replaced by a large air fluid level, with extension to right paracolic gutter, picture highly suggestive of EPN, class 3b
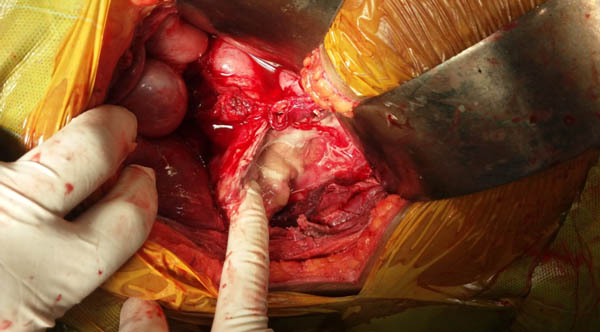
Initially, she was resuscitated with IV fluids, blood transfusion, insulin infusion and inotropes. Preoperatively, for three days, inj. Piperacillin-tazobactam, inj. Acyclovir 400 mg thrice a day and fluconazole 150mg/day were started which continued postoperatively for ten days. After optimisation, right nephrectomy was performed. Intraoperatively, renal capsule was densely adhered to liver, duodenum and ascending colon. Whole kidney was autolysed and replaced by pus except small upper pole [Table/Fig-6]. Histopathological examination showed, thrombosis of segmental renal vessels, infarction and necrosis with multiple neutrophilic infiltrate in renal parenchyma [Table/Fig-7a,b].
Intraoperative photograph showing renal capsule densely adhered to liver, duodenum and ascending colon. Whole kidney autolysed and replaced by pus except small upper pole.
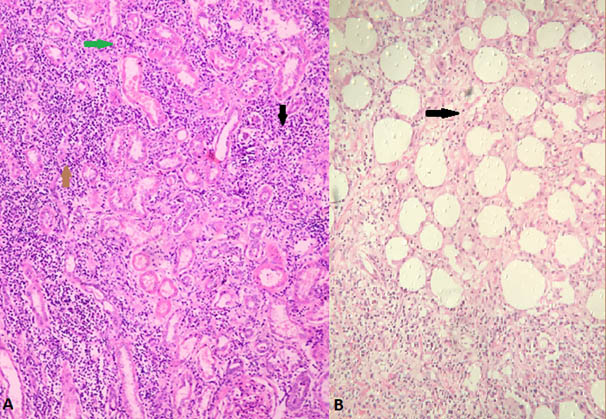
(a) Microscopic photograph (40X) H&E stained section shows dense polymorphonuclear infiltrate in the renal parenchyma (black arrow), infarcted glomerulus (green arrow) and tubules surrounded by dense neutrophilic infiltrate(brown arrow); (b) H&E (40X) stained section shows the polymorphonuclear infilterate seen extending into capsule and perinephric fat suggestive of emphysematous pyelonephritis (black arrow).
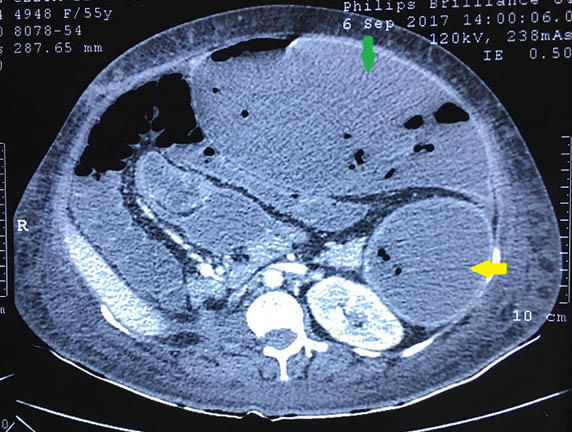
She was discharged after 3 weeks of hospitalisation. One month later, she developed splenic abscess which was percutaneously drained [Table/Fig-8]. Transthoracic Echocardiography did not reveal any vegetation. E.coli was grown in pus and managed by antibiotics according to sensitivity.
Follow-up 30 days postoperative, CECT Abdomen showing hypodense area seen in lateral aspect of spleen parenchyma with few foci of air likely Splenic Abscess with peripheral enhancement (yellow area); Left Kidney normal. Large well-defined collection seen along anterior abdominal wall communicating to splenic abscess, possibility of ruptured splenic abscess (green arrow).
Discussion
Emphysematous Pyelonephritis (EPN) is a rare, but severe, suppurative infection of the renal parenchyma characterised by gas formation in intra-renal and perirenal tissues by fermenting organisms [1]. The first case of gas-forming renal infection was reported by Kelly and MacCullum in 1898. Terms like ‘Renal emphysema’, and ‘pneumonephritis’ were used; ‘Emphysematous pyelonephritis’ was coined by Schultz and Klorfein in 1962 [2].
Diabetes mellitus is associated in upto 95% of patients [3] with female preponderance. The reported male: female ratio is 1:6. The mean age is 55 years (range 19-81). The typical presentations are fever (79%), abdominal flank pain (71%), nausea and vomiting (17%), dyspnoea (13%), acute renal impairment (35%), altered sensorium (19%), shock (29%) and thrombocytopenia (46%) [4]. Our patient presented with diabetic ketoacidosis, fever, right flank pain and shock. Predisposing factors are high tissue glucose concentration, gas forming organism, impaired vascularity, decreased immunity, and ureteral obstruction.
Escherichia coli, is the most common causative organism, accounting for 58-69% of the cases. Isolated rare case reports of Klebsiella pneumoniae, group B streptococcus, proteus, aspergillus, cryptococcus, amoeba, acinetobacter, actinomycosis, citrobacter, lactobacillus and rarely candida species have been reported. E. coli produce gases by fermentation of glucose and lactate. Candida species are also capable of producing gas by sugar fermentation [3,5,6]. Fungi can act as a sole or part of mixed infection, as in our patient. Gas analysis show nitrogen (60%), hydrogen (15%), oxygen (6.7%) and carbon dioxide (4.8%) with traces of ammonia, methane and carbon monoxide [3]. CECT of abdomen is diagnostic for EPN.
The possible mechanism of immune impairment in diabetics [7]:
Deficiency of the C4 component;
Decreased mobilisation of polymorphonuclear leukocytes, chemotaxis and phagocytic activity;
Inhibition of glucose-6-phosphate dehydrogenase (G6PD)- increasing apoptosis of polymorphonuclear leukocytes;
Glycation of immunoglobulin in proportion with the increase in HbA1c.
However, non-diabetic EPN patients show varying degree of immunologic impairment such as tuberculosis, AIDS and alcoholism [5]. Furthermore, immunosuppressive therapy amplifies the risk for development of EPN in transplant patients [8].
Three classifications have been proposed, but Huang and Tseng’s classification is commonly used that focuses on management and prognosis [Table/Fig-9] [3,5,9].
EPN classification system.
| Classification | Radiological basis | Class |
|---|
| Michaeli J et al., [9] | Plain radiograph and Intravenous pyelogram | I. Gas in the renal parenchyma or perinephric tissueII. Gas in the kidney and its surroundingsIII. Extension of gas through gerota’s fascia, or bilateral disease |
| Wan YL et al., [3] | CT | I. (Dry) Renal necrosis with presence of gas but no fluidII. (Wet) Parenchymal gas associated with fluid in renal parenchyma, perinephric space or collecting system |
| Huang and Tseng [5] | CT | 1. Gas limited in collecting system only2. Gas limited to renal parenchyma3A. Gas Extending to perinephric space3B Gas Extending to pararenal space4. EPN in solitary kidney or bilateral disease |
On extensive search, only one case of EPN with splenic abscess was found [10]. The index case is the first case of EPN caused by E. coli and Candida albicans with Herpes genitalis and oral candidiasis with subsequent development of splenic abscess, which was drained percutaneously. E.coli was grown in pus and managed by antibiotics. Thus, high index of suspicion is needed in un-controlled diabetic patients with pyelonephritis, since diabetes is a major risk factor for both EPN and splenic abscess.
Medical management comprises of aggressive resuscitation, antibiotics, control of blood sugar and electrolytes. EPN can be successfully treated with conservative and minimally invasive interventions. Ureteroscopic DJ stenting is indicated in dilated pelvicalyceal system. Percutaneous Nephrostomy (PCN) and PCD (percutaneous drainage) is helpful in extensive parenchymal involvement. Aggressive and early intervention will help to salvage the kidneys in class III and IV EPN. However, nephrectomy should be promptly attempted for patients not responding to conservative methods and patients with extensive, fulminant course of disease [11]. EPN was associated with a high mortality rate (78%), in last two decades. With improvement in management techniques, the overall mortality rate has reduced to 18% [3,12].
Conclusion
EPN is a fatal condition commonly seen in diabetics, and splenic abscess can complicate a patient’s clinical course. High index of suspicion required for diagnosis. Although uropathogens are the common aetiological agents, fungi can act as a sole or mixed aetiology. For extensive fulminating disease, aggressive resuscitation and nephrectomy provides rapid amelioration and prevent death.
[1]. Stein JP, Spitz A, Elmajian DA, Esrig D, Freeman JA, Grossfeld GD, Bilateral emphysematous pyelonephritis: a case report and review of the literatureUrology 1996 47(1):129-34.10.1016/S0090-4295(99)80399-9 [Google Scholar] [CrossRef]
[2]. Schultz EH, Klorfein EH, Emphysematous pyelonephritisJ Urol 1962 87:762-66.10.1016/S0022-5347(17)65043-2 [Google Scholar] [CrossRef]
[3]. Wan YL, Lee TY, Bullard MJ, Tsai CC, Acute gas producing bacterial renal infection: Correlation between imaging findings and clinical outcomeRadiology 1996 198:433-38.10.1148/radiology.198.2.85968458596845 [Google Scholar] [CrossRef] [PubMed]
[4]. Clark T, Fadul S, Abdalkareem M, Ali K, A case of emphysematous pyelonephritisJ R Soc Med 2009 102:75-77.10.1258/jrsm.2008.08021319208872 [Google Scholar] [CrossRef] [PubMed]
[5]. Huang JJ, Tseng CC, Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesisArch Intern Med 2000 160:797-805.10.1001/archinte.160.6.79710737279 [Google Scholar] [CrossRef] [PubMed]
[6]. Bhat RA, Bashir G, Wani M, Lone S, Emphysematous pyelonephritis caused by candida parapsilosis: an unknown etiological agentNorth Am J Med Sci 2012 4:364-66.10.4103/1947-2714.9952122912947 [Google Scholar] [CrossRef] [PubMed]
[7]. Peleg AY, Weerarathna T, McCarthy JS, Davis TM, Common infections in diabetes: pathogenesis, management and relationship to glycaemic controlDiabetes Metab Res Rev 2007 23(1):3-13.10.1002/dmrr.68216960917 [Google Scholar] [CrossRef] [PubMed]
[8]. Fujita S, Watanabe J, Reed A, Hemming A, Solis D, Netzel T, Case of emphysematous pyelonephritis in a renal allograftClin Transplant 2005 19:559-62.10.1111/j.1399-0012.2005.00264.x16008605 [Google Scholar] [CrossRef] [PubMed]
[9]. Michaeli J, Mogle P, Perlberg S, Hemiman S, Caine M, Emphysematous pyelonephritisJ Urol 1984 131:203-08.10.1016/S0022-5347(17)50309-2 [Google Scholar] [CrossRef]
[10]. Morioka H, Yanagisawa N, Suganuma A, Imamura A, Ajisawa A, Bilateral emphysematous pyelonephritis with a splenic abscessIntern Med 2013 52:147-50.10.2169/internalmedicine.52.830223291691 [Google Scholar] [CrossRef] [PubMed]
[11]. Rammohan T, Pandurangarao K, Santhosh B, Prasad DVSRK, Srinivas S, Sudharshan G, Management of emphysematous pyelonephritis: our experienceJournal of Evolution of Medical and Dental Sciences 2015 04(02):160-70.10.14260/jemds/2015/27 [Google Scholar] [CrossRef]
[12]. Aboumarzouk OM, Hughes O, Narahari K, Coulthard R, Kynaston H, Chlosta P, Emphysematous pyelonephritis: Time for a management plan with an evidence-based approachArab J Urol 2014 12:106-15.10.1016/j.aju.2013.09.00526019934 [Google Scholar] [CrossRef] [PubMed]