Tympanoplasty involves reconstruction of perforated tympanic membrane with or without ossiculoplasty [1]. It can be done both under local or general anaesthesia [2]. The main advantages of performing tympanoplasty under local anaesthesia are less bleeding, postoperative analgesia, faster mobilization of the patient, cost-effectiveness and the ability to test hearing intraoperatively [3-5]. However, inability to tolerate the discomforts (like a sense of noise, anxiety, dizziness, backache, claustrophobia or earache) and the possibility of sudden movement are the main concerns of not performing tympanoplasty under local anaesthesia [6].
MAC typically involves administration of local anaesthesia in combination with IV sedatives, anxiolytic and/or analgesic drugs which is a common practice during tympanoplasty [7]. A variety of drugs like Propofol, Benzodiazepines and Opioids have been used for this purpose however, none has been completely complication free. Over-sedation, respiratory depression, disorientation and hampered cooperation of the patient during surgery are the common complications reported [6,8,9].
Dexmedetomidine is a centrally acting selective alpha 2–receptor agonist having property of analgesia, sympatholysis and sedation in the titrated dose without major respiratory depression [10-12]. It is increasingly being used as a sedative-analgesic for MAC for various surgical procedures. Dexmedetomidine for MAC is often delivered as initial bolus dose range from 0.5-1.0 μg/kg over 10 -20 min followed by a continuous infusion of 0.2-0.7 μg/kg/hr [13]. Though there are many studies comparing Dexmedetomidine with other sedatives and analgesics (like Midazolam, Propofol, Fentanyl, and other opioids) for use during MAC [9,14,15], but very few studies have compared different doses of Dexmedetomidine to find out the optimal loading dose during MAC and especially so during tympanoplasty [16,17].
The purpose of present study was to compare the intraoperative analgesic and sedative efficacy of two different loading doses of Dexmedetomidine for tympanoplasty under MAC. The primary outcomes to be studied were quality of analgesia and sedation as well as patient satisfaction and surgeon satisfaction from two different loading doses of Dexmedetomidine. Their effects on vital parameters (BP, HR, RR and SpO2) and any intraoperative and post-operative complications like nausea, vomiting, respiratory depression, sedation and any other problem were also studied.
Materials and Methods
It was a randomised, prospective, double-blind study. After ethical committee approval from the institute, 80 patients of American Society of Anaesthesiologists (ASA) physical status grade I or II, age 16-65 years, weight 40-80 Kg scheduled for tympanoplasty under local anaesthesia between September 2015 to August 2016 were randomly selected in the ENT (Ear Nose and Throat) OT of the Institute.
All the patients were examined a day before surgery and thoroughly investigated according to the institute protocol. They were counselled with regards to sedation, local anaesthesia as well as the operative procedure and written informed consent was obtained from all the participants. Patients were instructed to keep fasting for 8 hours pre-operatively. Patients with known history of allergy to local anaesthetic, patient with seizure disorder, hypertension, cardiac disease, central or peripheral neuropathies or coagulopathies, pregnant and lactating females, patients receiving adrenoceptor agonist or antagonist therapy or chronic analgesic therapy were excluded from the study.
The VAS (0-10, where 0 indicated no pain while 10 corresponded to maximum pain), was explained to the patients during the pre-operative visit. Patients were randomly allocated into one of the two groups (n=40 each in Group I and Group II) using a computer generated random number tables. The drugs were prepared by a separate anaesthesiologist who was not involved in patient management or data collection and data was recorded by another observer who was blinded to group allocation. After arriving at the operating room, intravenous access was established. Patient’s Heart Rate (HR), Blood Pressure (BP), SpO2, Respiratory Rate (RR) and ECG monitoring were started and baseline parameters recorded. All patients were administered oxygen @ 4L/min. No sedative or premedication was used. Group I received intravenous dexmedetomidine 1.0μg/kg over 10 min and Group II received intravenous dexmeditomidine 0.5μg/kg over 10 min. Both were immediately followed by continuous maintenance infusion @ 0.4 μg/kg/h.
During this period, the patients were assessed every 5 minutes using Ramsay Sedation Score (RSS) (1 = agitated, restless; 2 = cooperative, tranquil; 3 = responds to verbal command while sleeping; 4 = brisk response to glabellar tap or loud voice while sleeping; 5 = sluggish response to glabellar tap or loud voice; 6 = no response to glabellar tap or loud voice). RSS of 3 was our target end point. If it was achieved before completion of loading infusion, then the infusion was stopped and noted. If any patient had sedation score <3 after completion of loading drug infusion then Midazolam 0.01mg/kg IV bolus was administered which was repeated if necessary till RSS was 3. In both the groups, the maintenance infusion was started immediately after stopping the loading infusion. After achieving RSS of 3, local anaesthetic was administered by the ENT surgeon using 6-8 ml of 2% lignocaine with adrenaline (1:20,000) in the incisura terminalis, post-auricular area and the four quadrants of the external auditory canal. Surgery was commenced after confirming adequate analgesia. Intraoperatively, HR, BP, RR and SpO2 were recorded every 5 minute during loading infusion of the study drugs and thereafter at 15-minutes intervals till the end of surgery. Sedation level (RSS) was assessed every 15 minutes and if RSS <3, IV Midazolam 0.01 mg/kg was administered as a rescue sedative in both the groups. If RSS >3 during intraoperative period, maintenance infusion was discontinued. Intraoperative pain intensity was evaluated every 15 min using VAS. If VAS >3, then IV Fentanyl in the dose of 1μg/kg was given as rescue analgesia. Total number of rescue doses of Midazolam and Fentanyl during surgery was recorded. The protocol was specified up to a maximum of three and four rescue doses of Fentanyl and Midazolam respectively. It was decided that at any time, if clinically indicated or if maximum doses of each of rescue drugs were already given, the study drug will be discontinued and the sedation technique will be converted to an alternative sedative/anaesthetic technique.
The maintenance infusion was discontinued approximately 20 minutes before end of surgery. Adverse events like hypotension (drop in SBP>20% of baseline or MAP <60 mmHg), hypertension (an increase in SBP or MAP >20% of baseline), bradycardia (HR <50 bpm), bradypnoea (RR <8 breaths/min), desaturation (SpO2< 90%), nausea, vomiting, dry mouth or any other event during or within two hours of the procedure were noted. A Numerical Rating Scale (NRS) with zero being least satisfied and 10 being most satisfied was used for grading of patient’s overall satisfaction with the procedure (as asked from patient on postoperative day 1) and a similar numerical rating scale (NRS 0-10) was used for grading of surgeon’s satisfaction with respect to surgical conditions and sedation technique [18]. Sample size calculation was based on power of 80% and an alpha-error of 5% based on a difference of two in patient satisfaction score between groups which showed that 40 patients per study group were needed.
Statistical Analysis
The statistical analysis was done using SPSS Version 15.0 statistical Analysis Software. Data were presented as number (%) and mean±SD (standard deviation). Student t-test was used to test the significance of difference between two means. To compare the change in a parameter at two different time intervals, paired t-test was used. The number of patients requiring rescue doses was analysed using Chi-square test. Confidence level of the study was kept at 95%; hence a P < 0.05 indicated a statistically significant association.
Results
No patients dropped out of the study. The two groups were comparable with respect to average age (p=0.596) and gender distribution (p=0.072) [Table/Fig-1]. There was no statistically significant difference in the baseline vitals between the two groups [Table/Fig-2].
| Group I (n=40) | Group II (n=40) | p-value |
|---|
| Age | 25.05±7.23 | 26.95±8.74 | 0.596 |
| Gender | | | |
| Female | 26 (65%) | 18 (45%) | 0.072 |
| Male | 14 (35%) | 22 (55%) |
Data are represented as mean, ±SD, n (%) and ratio. SD=Standard deviation
Baseline haemodynamic variables.
| Group I (n=40) | Group II (n=40) | p-value |
|---|
| Systolic BP | 121.20±10.30 | 122.08±7.98 | 0.672 |
| Diastolic BP | 71.00±8.74 | 74.43±7.22 | 0.060 |
| Mean BP | 87.73±8.95 | 90.31±6.87 | 0.153 |
| Heart rate | 74.88±7.14 | 74.30±7.75 | 0.731 |
| Respiratory rate | 13.00±0.93 | 13.38±0.84 | 0.062 |
| SpO2 | 99.00±1.34 | 99.45±1.01 | 0.094 |
Data are represented as mean±SD and ratio. SD=Standard deviation
Higher number of rescue doses of Fentanyl (2-3 doses) were required in higher proportion of patients of Group II (100%) as compared to Group I (15.00%) and the difference was statistically significant (Z =61.760;p<0.001) [Table/Fig-3].
Comparison between group of Rescue Dose of Fentanyl 1 μg/kg and Rescue dose of Midazolam 0.01 mg/kg.
| Group I (n=40) | Group II (n=40) | p-value |
|---|
| Number of rescue doses of Fentanyl |
| 0 | 1 (2.50%) | 0 (0.00%) | <0.001* |
| 1 | 33 (82.50%) | 0 (0.00%) |
| 2 | 6 (15.00%) | 19 (47.50%) |
| 3 | 0 (0.00%) | 21 (52.50%) |
| Number of rescue doses of Midazolam |
| 0 | 9 (22.50%) | 0 (0.00%) | <0.001* |
| 1 | 30 (75.00%) | 3 (7.50%) |
| 2 | 1 (2.50%) | 27 (67.50%) |
| 3 | 0 (0.00%) | 9 (22.50%) |
| 4 | 0 (0.00%) | 1 (2.50%) |
*=Significant (p=<0.05)
Proportion of patients requiring higher rescue doses of Midazolam (2-4 doses) was more in Group II (92.50%) as compared to Group I (2.50%) and the difference was statistically significant (Z =65.234;p<0.001) [Table/Fig-3]. Majority of patients (97.50%) of Group I required only 0-1 rescue doses of Midazolam.
Surgeon Satisfaction score of Group I (8.75±0.54) was found to be significantly higher than Group II (6.95±0.50) (Z=7.784; p<0.001) [Table/Fig-4]. Patient Satisfaction score of Group I (8.70±0.56) was also found to be significantly higher than Group II (6.28±0.60) (Z=7.914; p<0.001) [Table/Fig-4].
Comparison between group of surgeon satisfaction score and patient satisfaction score (NRS 1 – 10).
| Group I (n=40) | Group II (n=40) | p-value |
|---|
| Surgeon Satisfaction Score | 8.75±0.54 | 6.95±0.50 | <0.001* |
| Patient Satisfaction score | 8.70±0.56 | 6.28±0.60 | <0.001* |
*=Significant (p=<0.05)
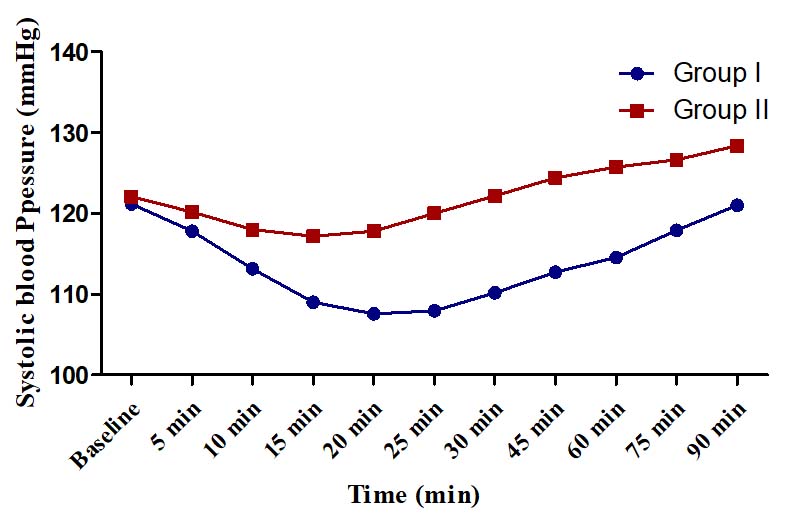
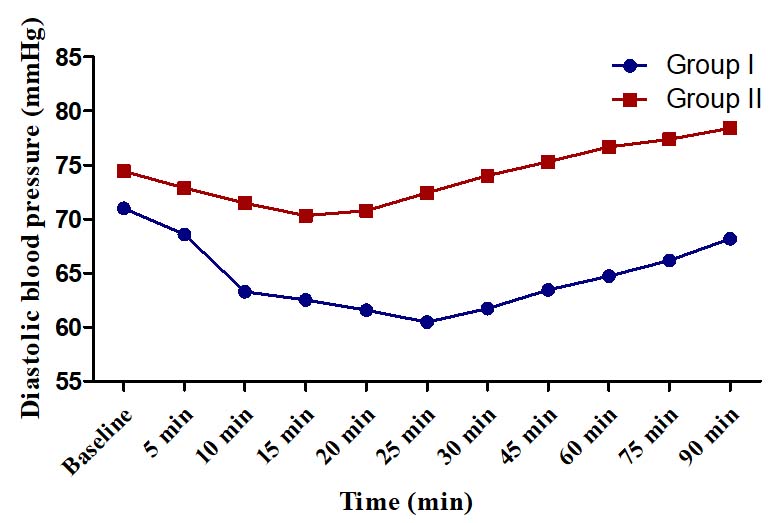
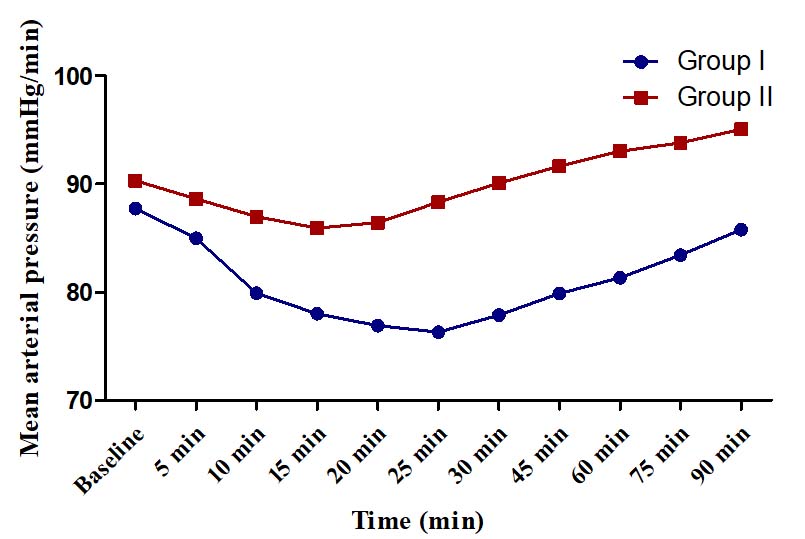
There was no hypotension or hypertension in any group. The changes in SBP, DBP and MAP were of modest degrees (<15%) in both the groups and did not warrant any corrective action. In Group I, maximum change in SBP from baseline was a decline of 13.60±4.22 mmHg (11.22%) observed at 20 min and in Group II, the maximum change was an incline of 6.33±3.20 mmHg (5.19%) at 90 minute [Table/Fig-5]. In Group I, the maximum change in DBP from baseline was 14.83% observed at 25 minute (decline of 10.53±3.19 mm Hg) whereas in Group II, the maximum change was found at 15 minute (decline of 4.10±1.74 mm Hg, 5.51%) [Table/Fig-6]. In Group I, the maximum change in MAP was a decline of 11.43±3.23 mm Hg (13.03%) observed at 25 min whereas in Group II, the maximum change was a decline of 4.76±2.23 mmHg (5.27%) [Table/Fig-7].
Systolic blood pressure (mmHg).
Diastolic blood pressure (mmHg).
Mean arterial pressure (mmHg).
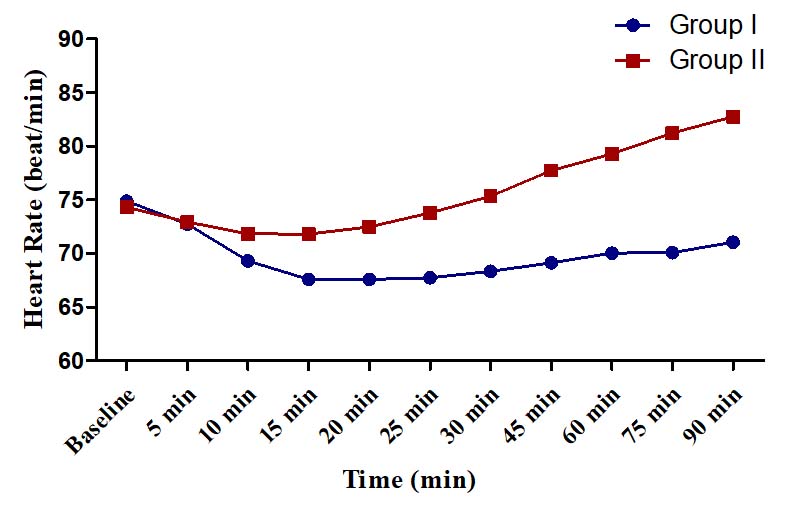
The changes in HR were of modest degrees in both the groups. In Group I, the maximum decline in HR was only 7.30±2.13 beats/min (9.75%) observed at 15 minutes [Table/Fig-8]. In Group II, the maximum decline was 2.50±3.04 beats/min (3.36%) found at 15 min and the maximum increment was 8.45±3.69 beats/min (11.37%) observed at 90 minutes [Table/Fig-8].
There was no significant respiratory depression requiring any intervention in any of the groups. The maximum decline in RR was 1% (seen at 25 min in Group I) [Table/Fig-9]. Mean saturation levels in both the groups were found to be between 99-100%. None of the patients had oxygen saturation level <95% at any period of observation.
Respiratory rate (per min).
Discussion
Dexmedetomidine has a novel property of providing “conscious sedation” and has been used as a single agent in many painful procedures [19-21]. Its sedative and anxiolytic effects result primarily from its activity in locus coeruleus of the brain-stem. It produces a modest reduction in Heart Rate (HR) and Blood Pressure (BP) ensuring a stable haemodynamic state [14,22].
The recommended loading dose of Dexmedetomidine has a wide variation ranging from 0.5 μg/kg to 1 μg/kg given over 10 to 20 minutes [13]. However, there is hardly any study to guide us whether to target for a lower or a higher loading dose of Dexmedetomidine in patients undergoing tympanoplasty under MAC. Present study was undertaken to evaluate the efficacy of two different loading doses of dexmedetomidine (0.5 μg/kg vs. 1 μg/kg) as sedative-analgesic in patient’s underoing tympanoplasty under MAC.
In a study by Verma R et al., Dexmedetomidine provided adequate sedation and analgesia with better patient and surgeon satisfaction when compared to propofol for tympanoplasty in LA [23]. Dexmedetomidine has also been found to provide qualitatively better sedation profile as compared to Midazolam-Fentanyl combination in patients for tympanoplasty under MAC [18]. Vyas DA et al., had also reported better surgeon and patient satisfaction with Dexmedetomidine than Midazolam in patients under MAC in ENT surgeries [24]. Though there are many studies in patients undergoing tympanoplasty under MAC showing superiority of Dexmedetomidine over Propofol, Midazolam or Fentanyl with regards to patient and surgeon satisfaction, but there are very few studies to find out the optimal dose of Dexmedetomidine required for this. In our study, patient satisfaction as well as surgeon satisfaction was more in group I as compared to group II. This was similar to the results of a previous study by Gupta P et al. which showed better patient and surgeon satisfaction with higher loading dose of 1.0 μg/kg as compared to 0.5 μg/kg [25]. However, their subjects included patients undergoing general and plastic surgery besides ENT surgeries.
In a study of MAC with Dexmedetomidine (DEX) by Candiotti KA et al., 326 patients were randomized 2:2:1 to DEX 0.5 μg/kg, DEX 1 μg/kg, or saline placebo initial loading dose, followed by a maintenance infusion of 0.2-1.0 μg/kg/hr of Dexmedetomidine (or equivalent volume of saline) titrated to a targeted level of sedation [26]. In this study, requirement of rescue dose of Midazolam and Fentanyl was higher in DEX 0.5 μg/kg group as compared to DEX 1 μg/kg group. Similar finding was present in which rescue doses of Fentanyl and Midazolam were required in significantly higher proportion of patients of Group II as compared to Group I. Ebert TJ et al. had also found progressive increases in sedation and analgesia with increasing plasma concentrations of Dexmedetomidine in their study on 10 healthy men [11]. Gupta P et al. had also found a greater requirement of rescue doses of Midazolam and Fentanyl in patients receiving Dexmedetomidine 0.5 μg/kg or normal saline as initial loading dose when compared to Dexmedetomidine 1.0 μg/kg [25].
In a study by Ebert et al., to see the response of increased plasma concentration of Dexmedetomidine on BP, there were dose dependent decreases in SBP, DBP and MAP [11]. Dose-dependent decreases in SBP, DBP and MAP were also present in the study by Kallio A et al., to see the effect of single intravenous doses of 12.5, 25, 50, and 75 μg in five healthy male volunteers [17]. Study by Gupta P et al., also showed significant reduction in MAP and fall in HR (15-20%) from the baseline values in Dexmedetomidine group [25]. This reduction was more obvious in group 1.0 mcg/kg which is similar to our study as well as studies by Ebert TJ et al., and Kallio A et al. However, the maximum decrease in HR was much less (<10%) in our study as compared to theirs (15-20%) with same loading dose of 1.0 μg/kg. Hall JE et al., had compared the safety and efficacy of two doses (0.2 Vs 0.6 μg/kg/hr infusion) of Dexmedetomidine in seven healthy young volunteers and found a 16% & 20% decrease in HR respectively during the 10 min. of initial dose [10].
Loading dose of 1μg/kg results in transient increase in BP and reflex decrease in HR. This is due to direct effect of α2 adrenoreceptor stimulation of vascular smooth muscle [11]. After the transient increase, it is followed by decrease in BP which occurs due to inhibition of sympathetic outflow that over rides the direct effect of Dexmedetomidine on vasculature. We did not observe the biphasic effect of Dexmedetomidine as we administered the loading dose of 1 μg/kg over 10 min. However there was a significant decrease in HR and mean BP after 20 minute of the loading dose. This may be due to the sympatholytic, vagotonic and baroreflex sensitivity reducing effect of Dexmedetomidine.
In present study, respiratory depression did not occur in any of the groups. This finding of our study is supported by various other studies [11,26,27]. Saturation levels in both the groups were found to be between 99-100%. None of the patients had oxygen saturation level <95% at any period of observation which is similar to the findings of other studies by Candiotti KA et al., and Mohamad MH et al., [26,27].
Limitation
Limitations of present study were that we had restricted our study only to patients undergoing tympanoplasty. Similar studies can be performed on a wider group of patients undergoing various other surgeries under MAC. The loading dose selected for the study were two extremes of the recommended dose range. In order to have a better idea of the optimal dose of Dexmedetomidine for MAC, patients can be allocated to a third group with an intermediate loading dose of 0.75 μg/kg and there can be further studies with variation in maintenance infusion dose as well. Moreover, assessment of pain and sedation are done using scores which are subjective. BIS monitor can be used to assess patient awareness for conducting similar studies in future.
Conclusion
Dexmedetomidine at loading dose of 1 μg/kg over 10 minute provides better sedation, analgesia, patient satisfaction and surgeon satisfaction as compared to loading dose of 0.5 μg/kg over 10 minute both being followed by same maintenance dose of 0.4 μg/kg/h. At this dose, Dexmedetomidine provides an adequate level of sedation and stable haemodynamics without causing any intraoperative or postoperative problem like nausea, vomiting or respiratory depression. So, Dexmedetomidine at this dose seems to be a safe and effective primary sedative technique for tympanoplasty under MAC.
Data are represented as mean, ±SD, n (%) and ratio. SD=Standard deviation
Data are represented as mean±SD and ratio. SD=Standard deviation
*=Significant (p=<0.05)
*=Significant (p=<0.05)