The diagnosis of meningitis is based on the documentation of an inflammatory response in the CSF along with additional tests that identify specific causative agents in the CSF. As prior antibiotic therapy can affect the bacteriological diagnosis from CSF (Gram stain and culture may be negative in up to 80% cases), and culture facility is not available at primary and secondary health care level, rapid bedside diagnostic tests like measurement of CSF CRP (latex agglutination or immunoturbidometric method) have been used in various studies [4-8]. CRP is an acute phase protein, the level of which is increased, in the blood and various other body fluids as a response to various non-specific stimuli (e.g., infection, inflammation, or tissue necrosis) [9]. It is also found to be a useful test in the diagnosis of partially treated pyogenic meningitis where CSF biochemical and cytological values may be equivocal.
In a recent study from Nepal, CSF CRP had a sensitivity and specificity of 96.87% and 74.73% for pyogenic meningitis; 66.66%, and 63.71% for partially treated meningitis; 20.58% and 50.94% for viral meningitis; 10% and 55.38% for tubercular meningitis, respectively [4]. In a study from India, CSF CRP test detected 80% cases of pyogenic meningitis, 15% cases of tubercular meningitis, and was negative in those without meningitis. The positive predictive value of the test for pyogenic and tubercular meningitis was 100% [7].
Most of the studies mentioned above were based upon CSF CRP estimation by latex agglutination (a semi-quantitative method), and very few of them mentioned the cut-off value for different types of meningitis. In the era of overuse of antibiotics, many of the pyogenic meningitis cases reaching to tertiary care hospitals have already received antibiotic therapy, thereby posing dilemma in interpreting the CSF test results. The present study was carried out to evaluate the usefulness of CSF CRP (by immunoturbidimetric method) in the rapid diagnosis of pyogenic (acute & partially treated) meningitis, and to differentiate the same from other forms of meningitis.
Materials and Methods
This cross-sectional study was conducted in the Paediatrics department of a tertiary care teaching institute in Eastern India over two year period from November 2013 to October 2015.
Inclusion criteria: Children aged 1 month to 14 years presenting with the symptoms (fever, headache, refusal to feed, vomiting, irritability, drowsiness, and convulsions) and signs (bulging anterior fontanelle, coma, and signs of neck stiffness) of meningitis were enrolled in a prescribed format and were examined. The prescribed/pre-designed format contained information on demographic parameters (age, sex, weight, height/length, and geographical location), neonatal details (weight, length, birth asphyxia, neonatal sepsis and/or jaundice), contact history with tuberculosis, clinical presentation (fever, headache, refusal to feed, vomiting, irritability, drowsiness, and convulsions), clinical examination findings of central nervous system (bulging anterior fontanelle, signs of neck stiffness, sensorium as assessed by Glassglow Coma Scale (GCS), cranial nerve palsy, motor and sensory system abnormality), other systemic examination abnormality (if any), previous admission details (hospitalisation, antibiotic and other treatment administration), immunisation details, history of drug ingestion or poisioning, laboratory parameters (complete blood count, renal and liver function tests, coagulation profile), and associated co-morbidity if any including HIV status (either of the child or the parents). World Health Organisation (WHO) growth chart was used for ≤5 years of age and Indian Academy of Paediatrics (IAP) gowth chart was used for >5-year-old children.
Exclusion criteria: Those having congenital malformations and non-infectious conditions of the CNS (epilepsy, drug or vaccine-induced problems, simple febrile seizures), HIV and other co-morbidities were excluded from study.
CRP estmation: Various methods have been used for estimation of CRP that include solid-phase monoclonal-polyclonal IRMA (Immunoradiometric Assay), immunoturbidimetric assay, latex-enhanced immunonephelometric test, and latex agglutination test [10]. The lower detection limit of CRP by solid-phase monoclonal-polyclonal IRMA (immunoradiometric assay) is 0.05 mg/L, by immunoturbidimetric assay is 0.1 mg/L, by latex-enhanced immunonephelometric test is 0.17 mg/L. As latex agglutination test is semi-quantitative, it has the lowest sensitivity. The immunoturbidimetric assay for CRP estimation was chosen because of its high sensitivity, and avilability in our set up.
Study procedure: Under strict aseptic precautions, 2 mL of CSF specimen was collected and sent for analysis (cytology, biochemistry, Gram stain and culture, and staining for acid-fast bacilli). CSF CRP measurement was done by a sensitive immunoturbidimetric assay (Integra 400 analyser; Hoffmann-La Roche). Taking CSF Gram stain and culture as gold standard tests, reference cases were categorised into five groups:, Group 0 (no meningitis/NM), Group 1 (bacterial meningitis/BM), Group 2 (partially treated meningitis/PTM), Group 3 (tubercular meningitis/TBM), and Group 4 (aseptic meningitis/AM). Written and informed consent from parent or legal guardian was obtained before enrollment into the study. The study was approved by the Local Ethics Committee, VIREC, Burla.
Sample size and sampling technique: A simple consecutive sampling technique was used. Assuming an overall prevalence of meningitis to be around 15% [1], precision level of 5%, the confidence level of 95%, and power of the study 80% through computer-generated software, minimum sample size calculated was 198 by using the n-master v2 (BRTC, Bagayam, Vellore).
Statistical Analysis
SPSS (version 24) and Microsoft Excel 2016 (student version) was used for data analysis. The data were expressed as the mean±SD. Test selection was based on evaluating the variables for normal distribution using the Shapiro-Wilk test. ANOVA and Tukey post-hoc test were used for comparision of group means, and to see the difference between them. A p-value <0.05 was considered to be statistically significant. Diagnostic Odds Ratio (DOR) was calculated to know whether CSF CRP estimation by immunoturbidimetric method was helpful in diagnosing different types of meningitis. Receptor Operating Characteristic (ROC) curve was plotted to know the cut-off value of CSF CRP in different subgroups of meningitis.
Results
Of 198 cases examined, 31 (15.65%) were bacterial meningitis, 61 (30.80%) were partially treated meningitis, 10 (0.5%) were tubercular, 57 (28.78%) were aseptic, and 39 (19.69%) were having no meningitis (controls). The demographic and other characteristics of study children have been described in the [Table/Fig-1]. The CSF characteristics of different groups of meningitis cases have been elaborated in [Table/Fig-2]. Of the 31 cases of bacterial meningitis, 25 cases were culture positive (blood=16, and CSF=9). The pathogens identified in blood cultures were: Staphylococcus aureus (7), Escherichia coli (3), Enterococcus (3), Acinetobacter (2), and Pseudomonas (1). The pathogens identified in CSF cultures were: Staphylococcus aureus (3), Streptococcus pneumonia (3), Escherichia coli (1), Enterococcus (1), and Acinetobacter (1). In all the 25 cases, the CSF cytology and biochemistry reports were suggestive of bacterial meningitis. In rest 6 cases, no organism was present either in blood or CSF. None of the partial treated meningitis cases showed any organism, however, the symptoms and CSF abnormalities were persisting. Of the 10 tubercular meningitis cases, all were having history of contact with adult tuberculosis cases. All had positive tuberculin tests (Mantoux tests), acid-fast bacilli could be identified in eight cases (CSF=4, gastric aspirate=4, both CSF and gastric aspirate=2), five cases had lung involvement (abnormal chest x ray), 3 had CT scan brain showing features of tubercular meningitis. Of 57 cases of aseptic meningitis, the CSF cytology and biochemistry reports were suggestive of aseptic meningitis in all the cases. PCR detected the viral agents in 31 cases: HSV I (25), HSV II (3), Measles (1), Varicella (1), and Dengue (1). Though enteroviruses are common cause of meningitis, the samples were not sent outside for testing of these viruses because of resource constraints. Distribution of CSF CRP values (mg/dL) in different groups of meningitis, outliers, and interquartile ranges in each group have been given in [Table/Fig-3].
Baseline characteristics of the study population.
| CharacteristicsN (%) | Pyogenicmeningitis(n=31) | Partially treatedmeningitis(n=61) | Tubercularmeningitis(n=10) | Asepticmeningitis(n=57) | No meningitis/controls(n=39) |
|---|
| Age <5 years | 18 (58%) | 35 (57%) | 6 (60%) | 25 (44%) | 18 (46%) |
| Male sex | 20 (65%) | 39 (64%) | 6 (60%) | 32 (56%) | 22 (56%) |
| Prior antibiotic administration | | 61 (100%) | 8 (80%) | 50 (88%) | 25 (64%) |
| History of contact with TB | 1 (3%) | 2 (3%) | 10 (100%) | 1 (2%) | 1 (3%) |
| Moderate to severe malnutrition (as per IAP growth chart** | 11 (35%) | 23 (38%) | 5 (50%) | 22 (39%) | 15 (38%) |
| Completely immunized | 22 (71%) | 45 (73%) | 7 (70%) | 41 (72%) | 28 (71) |
| Seriously ill at admission* | 5 (16%) | 7 (11%) | 2 (20%) | 7 (12%) | 3 (8%) |
*Defined by requirement of respiratory (non-invasive or invasive) and/or inotropic support
**IAP: Indian academy of Paediatrics
Cerebrospinal fluid parameters of different types of meningitis.
| Variables* | BM | PTM | TM | AM | NM | p-value** |
|---|
| Total count | 352±126 | 205±96 | 144±74 | 116±58 | 3±1 | 0.02 |
| Differential count |
| Polymorph | 86±10 | 64±21 | 09±07 | 11±08 | 0 | 0.01 |
| Lymphocyte | 14±10 | 33±21 | 90±07 | 88±08 | 05±12 |
| Protein | 68±26 | 54±22 | 204±95 | 35±16 | 27±11 | 0.01 |
| Sugar | 29±17 | 46±20 | 41±14 | 56±16 | 66±17 | 0.02 |
| Isolation of organism | N/A |
| Gram stain/AFB stain | +ve (25/31) | -ve (0/61) | +ve (8/10) | -ve (0/57) | -ve (0/39) |
| Culture | +ve (25/31) | -ve (0/61) | Not done | Not done | Not done |
*Expressed in mean±SD
**p-value determined by ANOVA test (<0.05 statistically significant)
Boxplot showing distribution of data in different subgroups of meningitis.
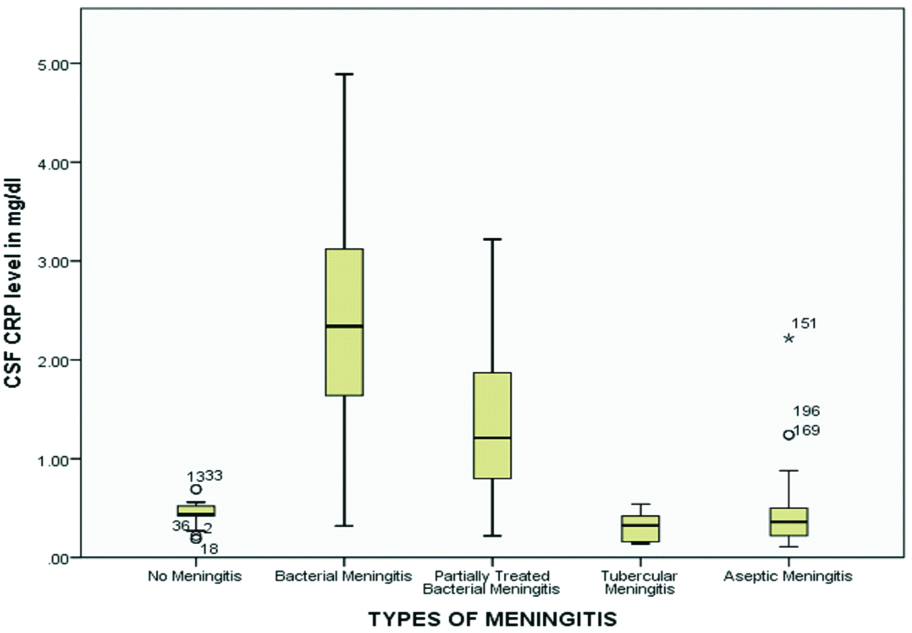
Inter- and intra-group comparison of mean CSF CRP level (mg/dL) was done in different groups of meningitis [Table/Fig-4]. There was a statistically significant difference in the mean CSF CRP level (mg/dL) in different groups as shown by one-way ANOVA {F (4,193) =59.47, p<0.001}. A Tukey post-hoc test revealed the mean CSF CRP level (mg/dL) of bacterial meningitis (2.32±1.14, p<0.001) and partially treated meningitis (1.33±0.72, p<0.001) to be significantly higher than that of no meningitis or control (0.44±0.11). The mean CSF CRP level (mg/dL) of partially treated meningitis (1.33±0.72, p<0.001), tubercular meningitis (0.31±0.15, p<0.001) and aseptic meningitis (0.43±0.35, p<0.001) cases was significantly more depressed than that of bacterial meningitis cases (2.32±1.14). The mean CSF CRP level (mg/dL) of tubercular meningitis (0.31±0.15, p<0.001) and aseptic meningitis (0.43±0.35, p<0.001) cases was significantly lower than that of partially treated meningitis (1.33±0.72, p<0.001).
ANOVA showing inter & intra group comparison of mean CSF CRP level between different groups.
| F (4,193)=59.470, p<0.001 |
|---|
| Groups | Mean±SD | | p-value |
|---|
| 0 (no meningitis) | 0.44±0.11 | Group 0 vs. Group 1 | <0.001* |
| 1 (bacterial meningitis) | 2.32±1.14 | Group 0 vs. Group 2 | <0.001* |
| 2 (partially treated meningitis) | 1.33±0.72 | Group 0 vs. Group 3 | 0.97 |
| 3 (tubercular meningitis) | 0.31±0.15 | Group 0 vs. Group 4 | 1.0 |
| 4 (aseptic meningitis) | 0.43±0.35 | Group 1 vs. Group 2 | <0.001* |
| Group 1 vs. Group 3 | <0.001* |
| Group 1 vs. Group 4 | <0.001* |
| Group 2 vs. Group 3 | <0.001* |
| Group 2 vs. Group 4 | <0.001* |
| Group 3 vs. Group 4 | 0.98 |
*p-value <0.05
The sensitivity, specificity, and diagnostic odds ratio (DOR) of CSF CRP was assessed in different groups of meningitis [Table/Fig-5]. The sensitivity and specificity was high in bacterial (sensitivity=87.1%, specificity=94.87%) and partially treated meningitis (sensitivity=88.52%, specificity=94.84%). A low sensitivity, but high specificity was found in tubercular (sensitivity=50%, specificity=96.85%) and aseptic meningitis (sensitivity=57.89%, specificity=95.12%).
Sensitivity, specificity & diagnostic odds ratio of CSF CRP in different groups.
| Group | Sensitivity (%)(95% CI) | Specificity (%)(95% CI) | Diagnostic Odds ratio (95% CI) |
|---|
| Bacterial meningitis | 87.1(71.1-94.8) | 94.87(83.1-98.6) | 142.7(28.1-725.6) |
| Partially treated meningitis | 88.52(78.2-94.3) | 94.84(82.8-98.48) | 124.87(21.31-731.94) |
| Tubercular meningitis | 50(23.6-76.0) | 96.85(83.2-98.7) | 18.5(2.80-122.1) |
| Aseptic meningitis | 57.89(45-69.8) | 95.12(83.11-98.58) | 25.44(5.58-115.95) |
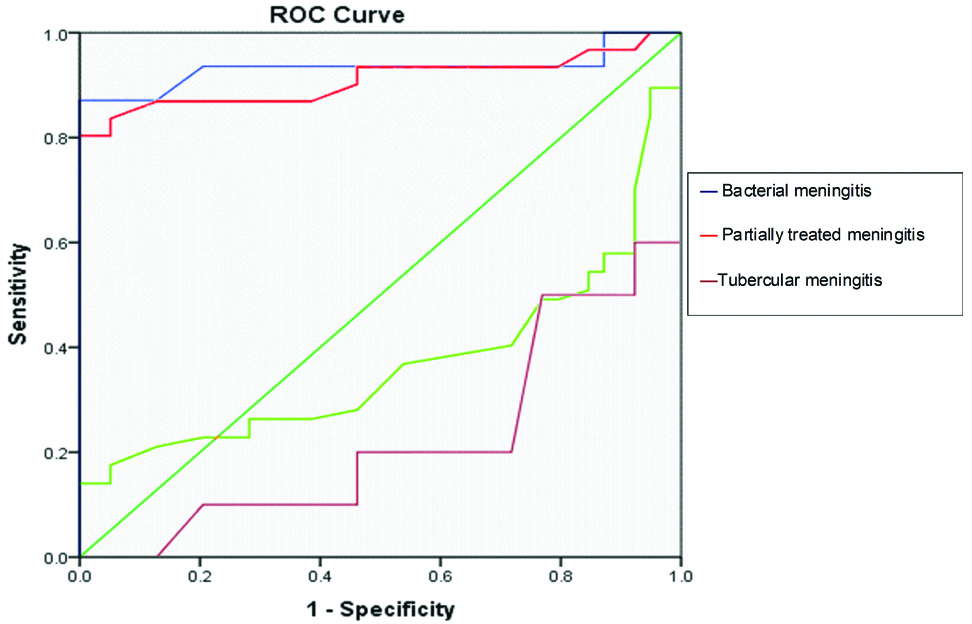
The sensitivity, specificity and area under curve (AUC) of CSF CRP was assessed in different groups of meningitis [Table/Fig-6]. A CSF CRP cut-off value of ≥0.62 mg/dL for bacterial meningitis, and a value of ≥0.55 mg/dL for partially treated meningitis were highly authenticated (as the AUC was >0.9). The CSF CRP cut-off values for diagnosing tubercular and aseptic meningitis were not authenticated (as the area under the curve was <0.5).
Showing sensitivity, specificity, AUC and p-value at different cut off value of CSF CRP.
| Group | CSF CRP Cut-off value (mg/dL) | Sensitivity (%) | Specificity (%) | AUC | p-value |
|---|
| BM | ≥0.62 | 87 | 95 | 0.933 | <0.001 |
| PTM | ≥0.55 | 87 | 88 | 0.909 | <0.001 |
| TM | ≥0.40 | 50 | 23 | 0.222 | 0.007 |
| AM | ≥0.735 | 14 | 100 | 0.369 | 0.030 |
BM: Bacterial Meningitis; PTM: Partially Treated Meningitis; TM: Tubercular Meningitis; AM: Aseptic Meningitis; AUC: Area Under Curve
There was no significant difference in the mean CSF CRP level (mg/dL) between no meningitis (0.44±0.11), tubercular meningitis (0.31±0.15, p=0.97), and aseptic meningitis (0.43±0.35, p=1.0) cases. There was no significant difference in the mean CSF CRP level (mg/dL) between tubercular meningitis (0.31±0.15, p=0.98), and aseptic meningitis cases (0.43±0.35).
ROC curves of different subgroups of meningitis have been shown in [Table/Fig-7].
ROC curve of bacterial meningitis.
Discussion
The present study showed a statistically significant difference in the mean CSF CRP level (mg/dL) in different groups of childhood meningitis that corroborates with previous study findings [4,7]. In one study, the mean(±SD) CSF CRP levels (mg/dL) for different groups of meningitis were as follows: bacterial meningitis=4.57±2.85, partially treated meningitis=2.31±2.39, tubercular meningitis=0.12±0.38, aseptic meningitis=0.45±1.69, and no meningitis=0.2±0.88 [4]. In another study, the levels were: bacterial meningitis=0.18±0.08, tubercular meningitis=0.08±0.03, aseptic meningitis=0.09±0.04, and no meningitis=0.06±0.03 [7]. The mean CSF CRP value in different groups in present study was far higher than one study [7], which may have been be due to mixing of partially treated with pure bacterial meningitis cases. However, the mean CSF CRP values in bacterial and partial treated meningitis in present study was little lower than the other study [4]. This may be because of the use of less robust method for CRP estimation in this study (latex aggplutination was used that might have given falsely higher levels) [4]. However, the mean CSF CRP values in tubercular and aseptic meningitis cases in present study were comparable to the findings of these studies. This may be explained by the fact that a less severe inflammatory response in later groups of meningitis might have caused lesser elevation of CRP with no significant difference in the methods used for estimation of CSF CRP.
The sensitivity and specificity of CSF CRP (immunoturbidimetric method) of all groups of meningitis cases in present study was not correlating with some of the previous studies [4,6,11]. As mentioned above, this variation in the sensitivity and specificity may have been be due to the different types of analyser used for CRP estimation (all these studies used the less sensitive latex agglutination test) in addition to the smaller sample size compared to present study. The diagnostic odds ratio (DOR) of CSF CRP (immunoturbidimetric method) was found to be highest for bacterial meningitis (142.7) followed by partially treated meningitis (124.87). The DOR was found to be the lowest for tubercular (18.5) and aseptic meningitis (25.44) cases. This means CSF CRP is a valuable diagnostic tool for diagnosing bacterial and partially treated meningitis compared to other types of meningitis. There have been no previous published studies estimating the DOR for CSF CRP (immunoturbidimetric method).
There was no significant difference in the CRP level between tubercular (0.31±0.15) and aseptic meningitis cases (0.43±0.35), this may be either due to a lesser/delayed inflammatory response to the antigens of corresponding organisms or may be due to a similar host response to this type of organisms (cell-mediated immunity plays an important role against both tubercular and viral antigens compared to the bacteria).
In the present study, we tried to find out the cut-off levels for diagnosis of various types of childhood meningitis. [Table/Fig-4] shows the Area Under Curve (AUC) for various types of meningits. It is clear from the table that, the AUC for TB meningitis and aseptic meningitis is less than <0.5 (50%), which means, the result would not be much significant in a clinical setting (though p-value is <0.05 showing statistical significance at cut-off levels). But, this needs to be validated in a larger sample of individual meningitis cases. Wide variation of CRP values in bacterial meningitis is also to be kept in mind while designing future studies.
Limitation
First, as it was a cross-sectional study, incidence rate could not be estimated. Second, being a tertiary care hospital based study, many partially treated meningitis might have prevented true classification of the meningitis cases. Third, CSF CRP with other valuable biomarkers like procalcitonin could not be comapared.
Conclusion
The sensitivity and specificity of CSF CRP for diagnosing bacterial and partially treated meningitis is quite high as compared to tubercular and aseptic meningitis. The cut-off value of CSF CRP for bacterial and partially treated meningitis was found to be ≥0.62 mg/dL and ≥0.55 mg/dL, respectively. Larger samples of individual meningitis cases are needed to validate the present CSF CRP cut-off values in future studies.
*Defined by requirement of respiratory (non-invasive or invasive) and/or inotropic support
**IAP: Indian academy of Paediatrics
*Expressed in mean±SD
**p-value determined by ANOVA test (<0.05 statistically significant)
*p-value <0.05
BM: Bacterial Meningitis; PTM: Partially Treated Meningitis; TM: Tubercular Meningitis; AM: Aseptic Meningitis; AUC: Area Under Curve