Ectopic pregnancy is a pregnancy where the fertilized ovum is implanted at a site other than the intrauterine cavity [1]. Studies in developing nations have reported mortality rates of about 1% - 3% which is nearly 10 times more than what is seen in developed nations [2].
The incidence of EPs is on the rise during recent times due to various factors like late primiparity, usage of intra-uterine contraceptive devices, smoking, increase in sexually transmitted diseases, use of emergency contraceptive pills like levonorgestrel, tubal sterilization and reversal of the same, previous history of abortions, previous pelvic surgeries and increase in the usage of assisted reproductive technologies [3].
A patient with an EP commonly presents with abdominal pain and bleeding per vaginum in the gestational age of 6 to 10 weeks. A transvaginal ultrasound is performed to ascertain the presence and site of the gestational sac. If the ultrasound is inconclusive, a serum beta-human Chorionic Gonadotropin (hCG) is assayed. A low beta-hCG most often indicates a failing/ abnormal pregnancy. Clinicians commonly wait for a window period of 48 hours to look for doubling of beta-hCG values. If the second assay after the window period does not show a sufficient rise in beta-hCG values and gestational sac is not visualized within the uterus in an ultrasound, then the woman is managed as a case of EP [5]. However, this time window required for serial measurement poses the risk of rupture in EP and the cost of performing an additional laboratory test. An early diagnosis thereby avoids unwarranted invasive surgery and facilitates for a conservative management instead [3].
This study was performed to assess the clinical presentation, beta-hCG values, mode of treatment and risk factors in relation to EP.
Materials and Methods
This hospital based observational, cross-sectional study was carried out at Sri Ramachandra Medical College Hospital, Chennai. The study was approved by the Institutional Ethics Committee of Sri Ramachandra University, Chennai and was performed in accordance with its recommendations. Records of confirmed EP patients admitted to the hospital from January 2014 to December 2014 were included in this study.
Women with normal pregnancy and non-viable intra-uterine pregnancy were excluded from this study. During the one year time frame of this study, a total of 128 EP cases were documented. Due to incomplete data being available in a few case sheets, only 116 cases were included in this study.
Charts were reviewed to determine patient symptoms, detailed history along with relevant laboratory investigations, ultrasound findings and mode of treatment. Beta-hCG was assayed on the Siemens ADVIA Centaur system based on a two-site sandwich immunoassay using direct chemiluminometric technology.
Statistical analysis was performed using Statistical Package for the Social Sciences software for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
A majority of the patients belonged to the age group of 20-35 years with around 49.2% within 26-30 years of age [Table/Fig-1].
Age group and risk factors of patients.
| Age in years | Percentage | Number of women |
|---|
| 15 – 20 | 2.3 | 3 |
| 21 – 25 | 18.8 | 22 |
| 26 – 30 | 49.2 | 57 |
| 31 - 35 | 21.9 | 25 |
| 36 - 40 | 7.8 | 9 |
| |
| Previous history of | Percentage |
| Assisted Reproductive Technologies | 20.3 |
| Abortions | 18 |
| LSCS | 16.4 |
| Ectopic Pregnancy | 14.8 |
| Salphingectomy | 14.8 |
| Sterilization | 13.3 |
| D&C | 4.3 |
| PCOS | 3.9 |
| Contraception | 3.8 |
| Diabetes Mellitus | 3.1 |
| Recannulisation | 3.1 |
| Seizures | 2.3 |
LSCS – Lower Segment Cesarean Section, D & C – Dilation And Curettage PCOS – Polycystic Ovarian Syndrome
The distribution of the various risk factors of EP in this study has been listed in [Table/Fig-1]. Assisted Reproductive Technologies (ARTs) like Ovulation Induction (OI), Intra Uterine Insemination (IUI), Intracytoplasmic Sperm Injection (ICSI) with Invitro Fertilization (IVF) were found to be the major risk factors associated with EP constituting 20.3%. Previous bad obstetric histories like abortions and previous EPs; previous surgeries like cesarean section, sterilization, recannulization and salphingectomy with/without oophorectomy formed the next set of risk factors ranging from 13-18%. Associated PCOS (Polycystic Ovarian Syndrome) was seen in less than 5% of these patients.
About one third of the patients presenting with EP were primiparous (38.6%) while the remaining patients were multiparous. While a majority of the patients presented with either bleeding per vaginum (61.7%), pain abdomen (18%) or both (14.8%), some patients were identified incidentally to have an extra-uterine growth during their Dating Scan (5.5%). Most commonly, the women presented at 7 weeks of gestation according to ultrasound and Last Menstrual Period (LMP). However, some patients reported as early as 4 weeks and even as late as 11 weeks [Table/Fig-2].
Beta-hCG levels according to weeks of gestation.
| Week of gestation | Number of cases | Beta hCG Mean (SD) mIU/mL |
|---|
| 4 | 7 | 3429(2254.7) |
| 5 | 11 | 2630(2387.3) |
| 6 | 21 | 5328.9(6382.3) |
| 7 | 30 | 8569.7(13319.7) |
| 8 | 20 | 16612.5(27249.6) |
| 9 | 15 | 3726.8(3049.2) |
| 10 | 9 | 37950.6(37950.6) |
| 11 | 3 | 1365.4(1731) |
A total of 74.3% of the patients had abdominal tenderness; cervical motion tenderness was seen in 43.7% cases and 47.8 % had adnexal tenderness. Around 36.4% of the patients had performed and found the urinary pregnancy test to be positive while the others had not performed it. A majority of the cases (69.7%) were found between September and December.
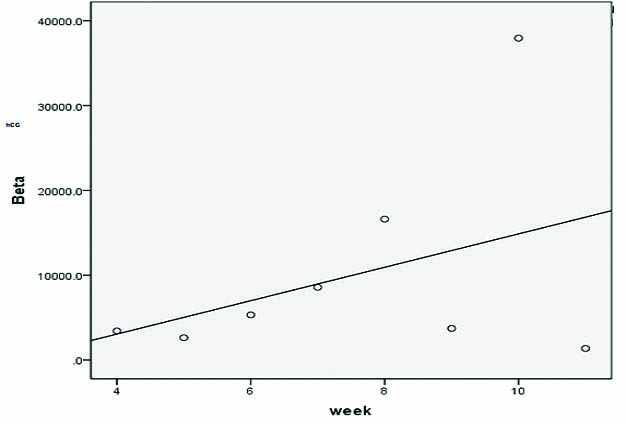
The mean Beta-human Chorionic Gonadotropin assayed on day one of presentation is tabulated according to the week of gestation in the following table [Table/Fig-2]. The range of beta-hCG values in EP according to the week of gestation is represented in [Table/Fig-3]. The reference intervals for hCG in normal pregnancy according to the week of gestation are also tabulated in [Table/Fig-3]. The Karl Pearson correlation co-efficient was determined between weeks of gestation and beta-hCG values assayed on day one of admission. There was a weak correlation (0.392) between the weeks of gestation and the beta hCG values [Table/Fig-4].
Range of values of beta-hCG based on gestational age in EP and the reference interval for normal pregnancy.
| Week of gestation | Range in EP in this study (mIU/mL) | Reference interval in normal pregnancy (mIU/mL) |
|---|
| 4 | 1174.7-6983.5 | 500-10000 |
| 5 | 32.4-6796.7 | 1000-50000 |
| 6 | 169.8-20636 | 10000-100000 |
| 7 | 113.3-54551.6 | 15000-200000 |
| 8 | 120.2-94933.4 | 10000-100000 |
| 9 | 243.5-10333.6 |
| 10 | 88.2-122604.5 |
| 11 | 342.6-3364 |
Correlation between weeks of gestation and beta-hCG values.
Ultrasound findings showed a live EP with gestational sac and yolk sac in 15.6% of the cases while only a mass was seen in 62.5%. Mass along with vascularity was noted in 11.7% of the cases, while free fluid was seen in 27.2%. Right-sided tubal ectopic pregnancies were seen in 58.4% of the patients and left side involvement was seen in 42.6%.
The most common mode of treatment was surgery (56.3%), medical treatment with multiple doses of methotrexate and folinic acid was carried out in 35.9% and the remaining 7.8% of cases were managed conservatively with observation for complete extrusion of products of conception spontaneously and supportive treatment for pain. Of the 72 cases that underwent laparotomy, 35.2% cases had a ruptured EP and 64.8% cases were of unruptured EPs; haemoperitoneum was observed in 10.2% of cases during laparotomy.
Unilateral Salpingectomy was the most common surgical management performed in 90.3%; salpingo-oophorectomy was done in 6.5% and salpingostomy in 3.2%. The modality of management according to the weeks of gestation at the time of presentation is tabulated below [Table/Fig-5]. Morbidity included anaemia (18%), blood transfusion in 14.8% and shock at presentation in 2.3% of the cases. No mortality was noted in this study period.
Modality of management according to weeks of gestation.
| Weeks of gestation | Number of cases n(%) | Medical Rx n(%) | Surgical Rx n(%) | Conservative Rx n(%) |
|---|
| 4 | 7(6) | - | 6(85.7) | 1(14.3) |
| 5 | 11(9.5) | 5(45.5) | 5(45.5) | 1(9.1) |
| 6 | 21(18.1) | 12(57.1) | 8(38.1) | 1(4.8) |
| 7 | 30(25.9) | 10(33.3) | 18(60) | 2(6.7) |
| 8 | 20(17.2) | 8(40) | 12(60) | - |
| 9 | 15(12.9) | 5(33.3) | 8(53.3) | 2(13.3) |
| 10 | 9(7.8) | 2(22.2) | 6(66.7) | 1(11.1) |
| 11 | 3(2.6) | 2(66.7) | - | 1(33.3) |
Discussion
In women presenting with first trimester bleeding, pain abdomen or both, the EP prevalence spans from 6 to 16% [6]. Most patients in this study fit into the age group of 25-30 years. Khaleeque F et al., also reported the same in their study [7]. The rate of EP was found to increase with age by Hoover KW et al., 0.3% in females aged 15-19 years and 1.0% in women aged 35-44 years [8].
In this study, 61.4% were multiparous and 38.6% were primiparous. Multiparous women were more predisposed to EP (61%) in the study conducted by Shraddha Shetty et al., [9]. The risk factors which have been found to be associated with EP seem to possess a common etiological factor: the hindrance of function of the fallopian tube. A compromised tubal ciliary motility or scarring due to previous surgeries or anatomic defects can restrict the fertilized zygote from passing through the fallopian tube into the uterus. This can lead to an implantation at a site prior to the defect, which can be functional or anatomic.
In this study, the most widespread predisposing factors were a history of infertility and assisted reproductive techniques, abortion, previous EPs and previous LSCS. Comparable risk factors were noticed in the study by Gupta R et al., [10]. These patients commonly presented with amenorrhea, pain abdomen and bleeding per vaginum. Abdominal tenderness, cervical motion tenderness and adnexal tenderness were the frequently seen clinical signs. An expedited diagnosis is made possible with the presence of these signs and symptoms.
A transvaginal ultrasound is performed to ascertain the location of the gestational sac. If the ultrasound is inconclusive, serum beta hCG is assayed. Serial measurement of beta-hCG may provide some clarity when the diagnosis is uncertain [11]. But in this study, there was a weak correlation (0.392) between the weeks of gestation and the beta-hCG values [Table/Fig-1]. However, this correlation cannot be interpreted to be statistically significant as: 1) there is a variation in the number of samples in each gestational age; 2) there are not enough samples in each category of gestational age; and 3) the standard deviation is so large. Therefore, beta- hCG may not be a reliable marker and newer biomarkers with better diagnostic capabilities are in need.
As of today, an amalgam of ultrasound and beta hCG is the approach to identify an extra-uterine pregnancy despite the fact that neither one on its own has been established as the gold standard for diagnosis of EP. An ultrasound, be it transvaginal or transabdominal, can recognize only around 20% of extra-uterine pregnancies, while the highly flaunted beta-hCG discriminatory zone gives the most elementary diagnostic assistance. In cases where the initial ultrasound is inconclusive, beta-hCG levels do not provide much direction as the discriminatory zone (the presumed beta-hCG level at which an intra-uterine pregnancy can be identified by an ultrasound) varies widely. It is anywhere between 1000 to 2000 mIU/mL, depending upon the hospital guidelines.
A beta-hCG level lesser than 1,500 mIU/mL is highly suspicious of EP at more than 8 weeks of gestation. However, an EP can occur at any level of β-hCG. This hormone is demonstrable in blood and urine as early as a week prior to an expected menstrual period. Serum levels as low as 2 IU/L can be detected, while urine levels as low as 20–50 IU/L can be detected [5]. While in the 4th week of an EP, the minimum and maximum values of beta-hCG were 1174 mIU/mL and 6983mIU/mL respectively in this study while the normal range for this week is between 500-10000mIU/mL. This wide range in the normal pregnancies itself makes the interpretation of beta-hCG difficult in EPs.
Similarly, the highest beta-hCG value in each category of EP was higher than the lower limit of beta-hCG in normal pregnancy. The number of EP cases with beta-hCG values overlapping the lower limit of normal pregnancy was 7, 6, 2, 4, 6, 1, 3 and 0 in weeks 4, 5, 6, 7, 8, 9, 10 and 11 respectively. The percentage of EP cases with beta-hCG values not overlapping with normal pregnancy were 0 %, 45.4%, 90.5%, 86.7%, 70%, 93.3%, 66.7% and 100% in 4, 5, 6, 7, 8, 9, 10 and 11 weeks respectively. It appears that a single value of beta-hCG cannot be of much use especially during the 4th and 5th weeks of gestation. This could be due to the wide range of beta-hCG values in normal pregnancy itself. During these early weeks, serial measurement to look for doubling time may be performed. From the 6th week onwards, predicting EP with a single measurement can be relied upon and it requires further validation.
Right-sided tubal EPs were seen in 58.4% of the patients and left side involvement was seen in 42.6%. In the 72 cases that underwent laparotomy, ruptured EP was present in 35.2 % of cases, 64.8% had unruptured EPs and haemoperitoneum was observed in 10.2% of cases. In the study by Latchow G et al., they showed that patients with a previous EP had a significantly higher risk of tubal rupture [12].
Knafel A et al., demonstrated that low haemoglobin and haematocrit values, along with higher gravidity can imply an augmented threat of tubal rupture [13]. In the current study, 61.4% were multiparous and 18% women were anaemic at the time of admission.
Morbidity included anaemia (18%), blood transfusion (14.8%) and shock (2.3%) while no maternal mortality was observed in the present study. By lowering and identifying risk factors and making a timely diagnosis, improvement in prognosis is likely [14].
The fatality of an EP multiplies with the chance of an impending rupture. It can detonate a potentially life-threatening intra-abdominal haemorrhage, cause shock and impair future fertility [15]. It is therefore, of ultimate importance to diagnose it much in advance for resolving the correct modality of treatment [11,16-21]. This can not only minimize the health care cost but also the mental agony of the patient.
Conclusion
Recognition of underlying risk factors, timely diagnosis and appropriate intervention will certainly abet in diminishing the morbidity and mortality related with EP, and can enhance the future reproductive outcome. The reference interval of Beta-hCG is wide in normal pregnancies itself. There is an overlap of beta-hCG values in EP with the lower limit of normal pregnancy especially so for the 4th and 5th week of gestation. Further studies with more number of women from the 6th gestational week onwards will aid in establishing the reliability of a single beta-hCG measurement in EP.
LSCS – Lower Segment Cesarean Section, D & C – Dilation And Curettage PCOS – Polycystic Ovarian Syndrome