CAPD is a well established treatment modality for ESRD patients which provides better mobility and independence to the patients. But the technique has few limitations in form of association with heightened risk of infection because of both, the dialysis procedure itself which has the potential for microbial contamination and the decreased immunity (both innate and acquired immunity) of the patient [1-3]. Peritonitis remains the major complication of peritoneal dialysis which leads to re-hospitalisation of the patient, removal of the catheter, shift to haemodialysis, and increased mortality [4-9]. Other infections, like exit site infections and tunnel infections, per se pose little risk but demand full meticulous care as these problems may lead to the development of peritonitis [10].
As recommended by the International Society of Peritoneal Dialysis (ISPD), the diagnosis of the peritonitis should be based on at least two of the following criteria: 1) clinical presentation suggestive of peritonitis, i.e., Abdominal pain and/or cloudy dialysis effluent; 2) Dialysis effluent white cell count >100/uL or >0.1×10-9/L (after a dwell time period of at least 2 hours), with more that 50% poly morphonuclear cells; and 3) Positive dialysis effluent culture [11].
FP though rare, is the most serious complication of peritoneal dialysis that carries high rate of morbidity and mortality. Even after the appropriate antifungal treatment, most of the time the outcome of the FP is not good, which is mainly due to the delay in the diagnosis. There is a need for surrogate markers which can help in establishing the diagnosis of fungal infection and also predict its outcome.
Peritoneal (1→3)-β-D-Glucan (BG) or Galactomannan (GM), both fungal cell wall components, are candidate biomarkers of FP. Detection of peritoneal GM and BG has been studied to diagnose the peritonitis due to Aspergillus niger and other fungi, with limited value for peritonitis due to Candida albicans. However this is in initial stages and their significance for diagnosis of FP needs to be further assessed. Polymerase Chain Reaction (PCR) has been reported to be a sensitive assay for early diagnosis of FP [12-14].
Materials and Methods
This was a ten year (2008-2017), retrospective study conducted on ESRD patients who were on CAPD at a tertiary care teaching hospital, located in South India, Andhra Pradesh. Case sheets were retrieved from the Medical records Department and the data was evaluated regarding demographic characteristics, cause of chronic kidney disease, presence of co-morbid illnesses, history of prior bacterial or fungal infection, nature of fungal isolates and the clinical outcome (which included mortality, shift to haemodialysis and re-insertion of the catheter). On the diagnosis of FP, the catheter was removed immediately and patient was treated appropriately.
Processing of the sample: Specimens included all the CAPD fluids and the CAPD catheters sent to the Department of Microbiology. Both types of specimens were processed for detection of fungi as well as bacteria.
Processing of peritoneal fluid: About 50 mL of Peritoneal Dialysis (PD) effluent was centrifuged at 3000 RPM (Revolutions Per Minute) for 15 minutes and the sediment was used to prepare Gram stain for detection of bacteria, yeast and yeast like fungi whereas 10% KOH mount of the sediment was used for detection of fungal elements.
Processing of catheter tip: Distal 2.5-5 cm of the CAPD catheter was cut from the catheter hub and dropped into a sterile container; catheter tip was flushed with sterile saline and was used for Gram stain and microscopy as above.
The sediment and the fluid from catheter tip was inoculated into two Sabouraud’s dextrose agar tubes of which one was incubated at 37°C and the other at 25°C. The cultures were followed upto a period of one month before being declared negative for any fungi. Any growth on SDA was identified based on macroscopic colony characters and lactophenol cotton blue mount microscopy findings in case of colony characters suggestive of filamentous fungus. Gram stain and Germ tube test were performed in case of growth suggestive of yeast and yeast like fungus. All the samples showing fungal elements in direct microscopy of the sample were repeated for confirmation and to rule out any contaminants and artefacts. Fungi were considered pathogens only on the presence of fungal elements in the KOH mount in repeated samples along with culture positivity on SDA.
All the samples were simultaneously processed for bacterial pathogen isolation and identification as per standard protocols [15].
Results
During the study period, 180 ESRD patients underwent CAPD among whom 18 (10%) developed FP. Characteristics of these 18 cases are described in [Table/Fig-1].
Culture status during the first and second attack and the outcome of the patient.
| S. No | Age/sex | Organism isolated | Out come |
|---|
| | First attack | Second attack | |
|---|
| 1 | 77/M | Klebsiella | Candida | Re-insertion |
| 2 | 74/M | Fusarium | - | died |
| 3 | 56/F | No growth | Candida | haemodialysis |
| 4 | 52/F | Curvularia | - | died |
| 5 | 48/F | E. coli | Candida | died |
| 6 | 69/F | Klebsiella | Candida | Re-insertion |
| 7 | 49/F | No growth | Candida | died |
| 8 | 65/F | Acinetobacter | Candida | haemodialysis |
| 9 | 72/M | Curvularia | - | died |
| 10 | 52/F | Pseudomonas | Candida | Re-insertion |
| 11 | 58/M | Aspergillus | - | died |
| 12 | 57/F | E. coli | Candida | haemodialysis |
| 13 | 45/F | No growth | Candida | Re-insertion |
| 14 | 67/M | Staph aureus | Aspergillus niger | died |
| 15 | 61/M | Candida | - | haemodialysis |
| 16 | 61/M | Pseudomonas | Candida | haemodialysis |
| 17 | 70/F | Pseudomonas | Aspergillus niger | died |
| 18 | 12/M | Pseudomonas | Aspergillus niger | haemodialysis |
M: Male, F: Female
Mean age of the patients (n=18) was 58 years (min-max 12-77 years) with female preponderance (n=10, 55.5%). Duration of CAPD before development of FP ranged from six months to 38 months. The predominant cause of ESRD was diabetic nephropathy (n=9, 50%). Other causes included Chronic Glomerulonephritis (n=6, 33.3%), Chronic interstitial nephritis (n=2, 11.1%) and autosomal dominant polycystic kidney disease (n=1, 5.5%). All the patients were hypertensive and a majority of them (n=11, 61.1%) also had diabetes mellitus. Presentation of FP was cloudy lysate and abdominal pain (n=17, 94.4%), nausea and vomiting (n=11, 61.1%) and fever (n=7, 38.8%).
From the 18 patients, in five patients (27.7%) fungus was isolated in the first attack of peritonitis (primary FP) without any previous history of bacterial peritonitis or antibiotic intake, whereas the other 13 patients (72.2%) had history of antibiotic intake, subsequent to bacterial peritonitis or culture negative peritonitis, within 2-3 months of onset of FP (secondary FP). Of these 13 patients three patients (16.6%) had the history of culture negative peritonitis and 10 patients (55.5%) had history of primary bacterial peritonitis. Majority of the bacterial pathogens (90%, n=9) were identified as gram negative bacilli.
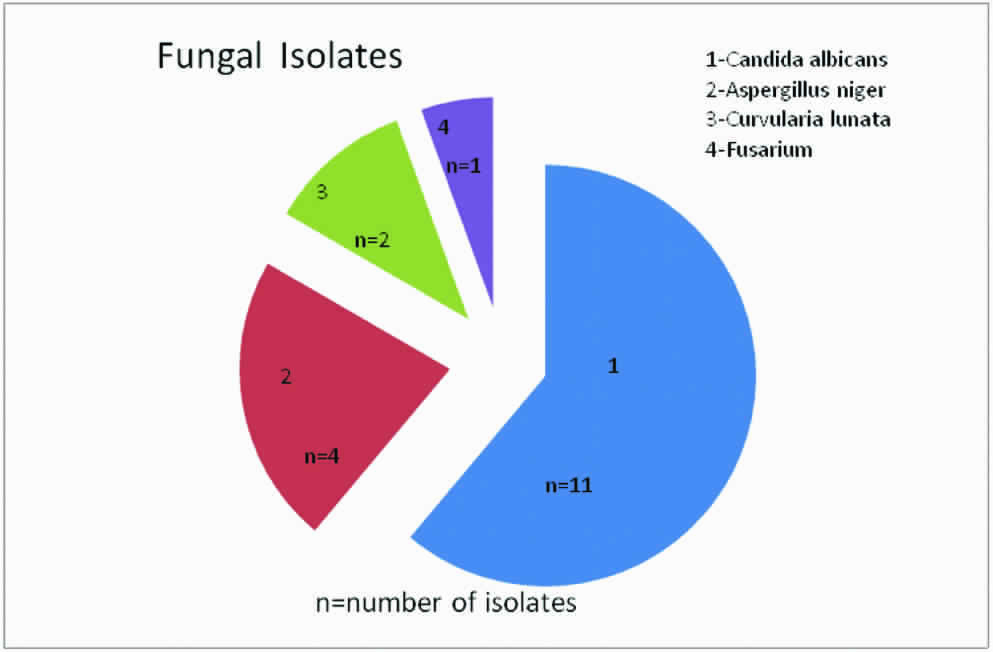
Among the fungal isolates (N=18), most common was Candida albicans (n=11, 61.1%) followed by Aspergillus niger (n=4, 22.2%), Curvularia lunata (n=2, 11.1%) and Fusarium (n=1, 5.5%) in descending order as shown in [Table/Fig-2].
Showing different fungi isolated.
Re-insertion of CAPD catheter was done in 4 (22.2%) patients, 6 (33.3%) were shifted to maintenance haemodialysis and 8 patients (44.4%) expired during the episode of FP. Data shown in [Table/Fig-3,4].
Risk factors and poor outcome (technique failure and mortality).
| Variable | | Recovery (n=4) | Poor out come (technical failure/Expired) (n=14) | p-value |
|---|
| Age | <50 | 1 | 3 | 0.99 |
| >50 | 3 | 11 | |
| Sex | Male | 1 | 7 | 0.58 |
| Female | 3 | 7 | |
| Diabetes Mellitus | Present | 4 | 7 | 0.11 |
| Absent | 0 | 7 | |
| Culture | Candida | 4 | 7 | 0.11 |
| Filamentous fungus | 0 | 7 | |
| Previous antibiotic exposure | Present | 4 | 9 | 0.59 |
| Absent | 0 | 5 | |
Risk factors for mortality.
| Variable | | Re-insertion and haemodialysis (n=10) | Expired (n=08) | p-value |
|---|
| Age | < 50 | 2 | 2 | 0.85 |
| >50 | 8 | 6 | |
| Sex | Male | 4 | 4 | 0.95 |
| Female | 6 | 4 | |
| Diabetes Mellitus | Present | 4 | 7 | 0.11 |
| Absent | 6 | 1 | |
| Culture | Candida | 9 | 2 | 0.02 |
| Filamentous fungus | 1 | 6 | |
| Previous antibiotic exposure | Present | 8 | 6 | 0.75 |
| Absent | 2 | 2 | |
Discussion
Peritonitis is an important complication among CAPD patients leading to hospitalisation of the patient, increased morbidity, catheter removal, shifting to haemodialysis and high mortality rate. Peritonitis can result from various conditions like touch contamination, catheter related problems, gastrointestinal and gynaecological problems and from sepsis.
Although FP is a rare complication among CAPD patients its incidence varies from 3-30%. The incidence of FP in present study was 10%, which is in concordance with the other studies [4-9]. Many factors such as decreased cellular immunity, antibiotic therapy following bacterial infection, rupture of cutaneous barrier and prolonged hospitalisation have been postulated to favour the occurrence of FP.
FP is difficult to diagnose due to the non-specific signs and symptoms which mimic bacterial peritonitis. In a symptomatic patient, repeated culture negative results and failure of antibiotic therapy may indicate FP. Repeated microscopic examination of dialysate fluid can be helpful in diagnosis of FP [12].
In present study maximum number of patients (n=11, 61.1%) were in the age range of 50-70 years. Old age as a risk factor has been debated in the studies, with reports, both in favour and against it [16,17].
The role of diabetes mellitus in development of FP in CAPD patients is still debatable as studies favouring as well as refuting it are available in literature. Studies supporting the role of diabetes mellitus in development of FP recommended strict glycaemic control for prevention of peritonitis in CAPD patients [18-20]. In present study 11 patients (61.1%) had diabetes mellitus.
According to observations by different authors the sensitivity of gram stain and microscopy varies widely, ranging from 10-70%. Despite the limitations, Gram stain and direct microscopy are considered to be very useful diagnostic tool in case of FP. The gram negative and gram positive bacteria as well as yeast and yeast like fungi can be detected easily and quickly by gram stain while direct microscopy with 10% KOH helps in detecting filamentous fungi. Gram stain findings can be helpful in taking the decision of prompt removal of the catheter, which is an important prerequisite for treatment of FP. The identification of Candida species is important for specific antifungal therapy but culture results and availability of antifungal susceptibility may take some time which can have definitive consequences in terms of outcome. However culture is the gold standard for diagnosis of FP [21,22].
Patient was empirically treated as per ISPD 2016 guidelines. After initial report, antifungal was changed to Voriconazole, if filamentous fungus was isolated [11].
It has been postulated that fungal infection appears mostly after episodes of bacterial peritonitis due to gram negative bacilli. Antibiotic administration in the previous months for the treatment of bacterial peritonitis episode is closely related to FP. It has been suggested that antibiotic therapy destroys the normal bacterial flora of the colon which provide protection by several mechanisms one of them being competitive exclusion. This leads to the overgrowth of intestinal fungi promoting future migration into the peritoneal cavity by routes not well defined and thus causing FP. Candida is normal flora in the gut which can overgrow after suppression of normal flora [23,24].
Reported incidence of prior antibiotic exposure in CAPD patients with FP ranges from 34% to 80% [25-27]. In this study 72.2% (n=13) of patients had prior exposure to antibiotics, while 27.7% (n=5) of the patients did not receive any antimicrobial therapy, this indicates the role of several other factors for development of FP. The causes of these de novo cases of FP may include direct contamination of the dialysis catheter during the exchange procedure, underlying intestinal pathology such as diverticulosis in host and environment contamination [28].
Yeast and yeast like fungi are reported to be the predominant causes of FP but filamentous fungi are also increasingly being reported. Candida species are the commonest fungi, responsible for about 60-90% of all cases of FP [4-9]. Candida albicans has been considered as the predominant species. But over the last decade non-albicans species, other yeast and filamentous fungi are increasingly being reported with increased mortality rate. Similar to yeast the filamentous fungi or moulds are widely prevalent in nature. Though described in much lower percentage than yeast, they have sparked clinical interest as the causative agents for the FP, since they are more resistant to antifungal agents [29].
Candida albicans (n=11, 61.1%) remains the most common cause of FP in this study which is in concordance with the other studies, remaining others 38.8% (n=7) were filamentous fungi such as 22.2% (n=4) Aspergillus niger, 11.1% (n=2) Curvularia lunata and 5.5% (n=1) was Fusarium spp.
The Peritoneal Dialysis catheter should be removed as soon as possible after diagnosis of FP as it has been suggested that fungi form a biofilm on the surface of the catheter which reduces the penetration of antifungal agent, rendering the eradication of fungal infection difficult without the removal of catheter [30,31].
Most of the studies consistently indicate that leaving the catheter in situ is associated with greater mortality [18-23]. One Indian study reported mortality rate of 60.46% in which peritoneal catheter was left in situ, in most of the patients [16]. Another study reported 20% mortality rate due to FP, 60% patients recovered completely and were initiated on CAPD and 20% of the patients were shifted to haemodialysis due to technique failure [27]. In this study CAPD catheter was removed immediately after the diagnosis of FP and patients were treated with appropriate antifungal therapy. Re-insertion was done in 22.2% (n=4), 33.3% (n=6) of the patients were shifted to haemodialysis therapy. Eight (44.4%) patients expired despite the timely removal of the catheter and initiation of antifungal therapy. It was also seen that 75% (n=6) of the mortality was in patients with filamentous fungus, depicting the aggressiveness and resistance of the filamentous fungi to the antifungal treatment.
In another study it was reported that the isolation of fungus or gram negative bacteria was considered as the primary predictor of adverse clinical outcomes, and was recommended the early catheter removal and appropriate antimicrobial therapy. Every effort must be made to identify the causative organism responsible for peritoneal dialysis associated infection [32]. A high index of clinical suspicion; timely diagnosis and institution of appropriate antifungal treatment are essential for successful management of FP in CAPD patients.
Conclusion
Candida species remain the most common cause of FP in present study but filamentous fungi were more dangerous and responsible for most of the mortality.
M: Male, F: Female