Pupil dilatation is essential in routine eye examinations, to facilitate evaluation of the posterior segment by the eye care practitioners. It is a prerequisite in many ocular surgeries such as cataract, posterior segment surgeries and in specialized outpatient procedures such as posterior segment laser treatments. Various dilating agents have been tried and evaluated, but the combination of Tropicamide with Phenylephrine has proved to be the most effective [1]. Ever since, it has enjoyed worldwide popularity as a powerful and rapid mydriatic drug for almost 40 years, mainly because of its suitable duration of action and minimal side effects [2].
Phenylephrine is an agent to dilate the pupil, which belongs to the phenethylamine class. It is a selective α1-adrenergic receptor agonist. Tropicamide is muscarinic antagonist that produces short term mydriasis and cycloplegia. The use of the sympathomimetic drug causes the iris dilator muscle to be directly stimulated, causing increased dilation [3]. Combination of formulations with Tropicamide and Phenylephrine have been proven to be effective and has been widely used for the past four decades, and has been commonly used for all ophthalmological screening processes or surgeries [4,5]. In the United States, the sympathomimetic drop most commonly used along with Tropicamide is 2.5% Phenylephrine hydrochloride. In India, most of the commercially available drops have 0.8% Tropicamide with 5% Phenylephrine.
The rapidity of the onset of the action and the estimated time of adequate pupil dilatation, when known accurately, will help the clinical work flow of patients with regards to the waiting time. The racial properties determining the pigmentation of the iris also determines the rate and extent of pupil dilatation due to the property of the drug adhering to the melanin pigments. Tropicamide has failed to produce satisfactory mydriasis when given alone, especially in patients with darkly pigmented irides or with diabetes mellitus [6,7]. This study was undertaken to look at the above mentioned properties of the pupil in an Indian Population.
Materials and Methods
This was a prospective observational study conducted at the Department of Ophthalmology, at a tertiary care centre in Southern India from March to April 2015. The study was approved by the Institutional Review Board and Ethics Committee {IRB Min No: 9301 (observe) dated 05/02/2015}.
All patients between 18 to 50 years, attending the out-patient clinic during the study period were included after informed consent. Patients with shallow anterior chamber, previous ocular surgery/laser, pseudoexfoliation, past ocular trauma, uveitis, presence of synechiae, raised IOP>25 mmHg, anisocoria, hypertension or Ischemic Heart disease, usage of any topical eye drops in the last one month or known allergy to any of the drugs were excluded from the study. The right eye of the patient was chosen as the study eye. For one eyed participants, the functioning eye was chosen as the study eye.
The sample size was calculated based on the study done by Jethani J et al., [2]. Keeping the Type 1 error as 80, Type 2 error as 5, baseline pupil size as 2.1 mm and post dilated diameter as 2.75 mm, to study the effect size of 0.25 mm difference in pupil size (which is 10% of the baseline value), the required sample size to study was 128 eyes (128 patients) using the formula Single Mean-Paired t-test.
The first two patients registering at the outpatient clinic at each hour satisfying the inclusion and exclusion criteria were enrolled into the study. All participants underwent a thorough undilated slit-lamp examination and a baseline measurement of the diameter of the pupil with the Optical Biometer (OB) before the instillation of the dilating drop by the primary observer (HA). The Pupil Dilatation (PD) was measured by NIDEK Optical Biometer (NIDEK CO., LTD., Japan). The participants were seated comfortably with the chin resting on the chin rest and left eye fixed on the target and the machine took a series of readings as per the protocol, the final displayed reading was taken as the study value. Baseline PD and all the follow up reading were measured with the OB, with room illumination on and OB light off (0.34 fc or 3.6 lux as measured with Seculife Illumination Meter, Class B per DIN 5032-7, Gossen Metrawatt Ltd.,). This protocol was set as a default due to logistic consideration.
Full dilatation or end point dilatation was defined in present study as three consecutive pupil size readings measured within 0.1 mm of each other and no visible constriction of pupil to a bright light source (Indirect ophthalmoscope light). Pupil constriction was looked at only after endpoint dilatation was achieved.
Following the baseline measurement, a single drop of Tropicamide (0.8%) with Phenylephrine (5%) was instilled in both eyes as part of the routine examination. The subjects were asked to keep the eye closed gently for a minute. To standardize the size of the drop instilled, all bottles were pierced with the same gauge (26 G) needle. PD was measured starting from two minutes after instillation of the dilating drop. Thereafter, pupil size was measured, every 2 minutes till 30 minutes post instillation of the dilating drop. After 30 minutes, PD was measured for every two minutes till the endpoint dilatation or 44 minutes after drug instillation. These measurements were done by the second observer (SER). Details from the OPD records were collected at the end of the clinic after the examination by the doctors, to ensure that the patients fulfilled the inclusion and exclusion criteria.
The onset of dilatation was defined as the reading when the PD was at least 10% more than the baseline diameter. The amplitude of dilatation was calculated as the difference in the PD between the baseline and the final endpoint diameter. The time taken for full dilatation was calculated from instillation of drops till the patients reach the end point. The rate of dilatation was calculated by taking the amplitude of dilatation divided by time taken for full dilatation.
Statistical Analysis
Data were summarised using mean (standard deviation), for continuous variables, frequency (%) for categorical variables. Data were screened for outliers and extreme values using Box-Cox plot and histogram (for shape of the distribution). Summary statistics were used for reporting demographic and clinical characteristics. A t-test used for analysis of continuous data with Normal distribution and Mann-Whitney U test for data with non-Normal distribution with Dilatation. ANOVA was used to compare more than two groups (Baseline PD and Amplitude). Differences were considered significant at p<0.05. All the statistical analysis was performed using SPSS 16.0.
Results
A total of 132 participants were recruited for the study after informed consent. Four patients were excluded from the study after consenting as one subject had uveitis, one patient was on topical eye drops (which was picked up from the OPD records) and two patients withdrew from the study before the complete set of readings were taken; 128 patients remained for final analysis.
Of the 128 participants, 2 participants did not achieve an end point dilatation at the end of the study time (44 min). These two participants were excluded from the calculation for amplitude and time taken for maximal dilatation, but were included for the analysis of rate of dilatation (126 eyes). The patients were sub categorized into 4 groups based on their baseline PD: Up to 4, 4.1 to 5, 5.1 to 6 and > 6 mm. Pupil characteristics and its various means of the above subgroups are given in [Table/Fig-1].
Mean value of the parameters and dilating characteristics of the pupil.
| Sub Groups (n=126) | Baseline PD withroom illuminationin mm(Mean±SD) | Onset of dilatationin minutes(Mean±SD) | Time taken forfull dilatation inminutes(Mean±SD) | Amplitude in mm(Mean±SD) | Time taken for fulldilatation fromonset in minutes(Mean±SD) | Rate of dilatationin mm/minute(Mean±SD) | Number ofpatients in eachsub-group |
|---|
| Upto 4 mm | 3.60±0.20 | 4.29±2.69 | 35.43±4.42 | 3.07±0.60 | 31.14±6.62 | 0.086±0.014 | 7 |
| 4.1 to 5 mm | 4.60±0.23 | 6.53±4.07 | 33.76±5.07 | 2.73±0.55 | 27.22±4.63 | 0.082±0.020 | 49 |
| 5.1 to 6 mm | 5.50±0.24 | 9.41±4.30 | 32.73±4.80 | 2.28±0.55 | 23.30±5.96 | 0.071±0.020 | 44 |
| > 6 mm | 6.50±0.35 | 14.1±4.30 | 31.46±5.55 | 1.71±0.48 | 17.15±7.42 | 0.055±0.019 | 26 |
| p-value | <0.05 | <0.05 | 0.16 | <0.05 | <0.05 | <0.05 | 126 |
* SD: Standard Deviation, mm: Millimetre
Except for time taken for full dilatation, the other parameters studied were statistically different from each other. The average onset of dilatation was at 9.02±5.2 minutes (2 to 24 min) from the time of instillation of drops. The mean time taken for full dilatation was 33.02±5.09 minutes (22 to 42 min). The mean amplitude of dilatation was at 2.39±0.68 mm (0.9 to 4.2). The rate of dilatation of the pupil was 0.07±0.022 mm/sec (0.03 to 0.14 mm/min). The average PD of the participants at 10, 16 and 20 minutes was 6.2, 6.8 and 7.1 mm respectively [Table/Fig-2].
Bird’s eye view of the graph displaying the baseline pupil diameter to the different pupil dilating characteristics (N=126 patients). Baseline pupil diameter and amplitude in millimetres, Onset and time taken for dilatation in minutes and the rate of dilatation in millimetres/minute.
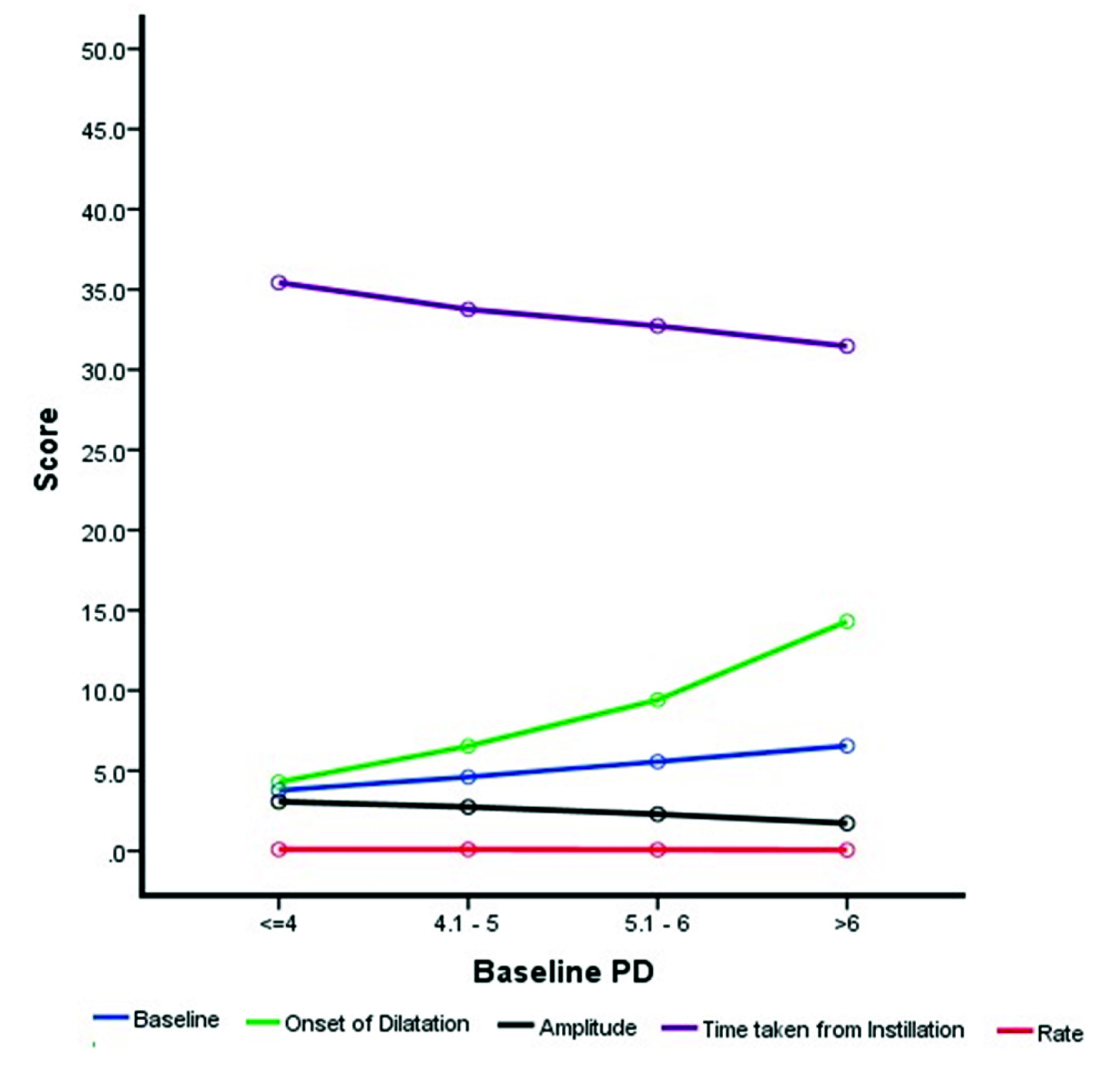
A total of 98.4% of the eyes achieved maximal dilatation as per our definition. The average time taken for dilation was 33.03 minutes (22 to 42 min). The average maximal PD was 7.5mm among the 126 patients who achieved dilatation according to the study criteria. Among the 126 participants, 94 participants had 3 consecutive readings which read the same and pupil did not react to the bright source of light. The remaining 32 participants had three readings which were within 0.1 mm of each other; their pupils too did not react to the bright source of light at the third reading. Statistically significant differences in pupil characteristics between the groups were seen in onset of dilatation, time taken for full dilatation and rate of dilatation. The characteristics and the parameters with their mean average are displayed in [Table/Fig-3].
Data of the baseline, time taken and the final pupil characteristic of the study population.
| No: of patients (n = 126) | Baseline pupil diameter with room illumination in mm in 3.6 lux (Mean±SD) | Onset of dilatation in minutes (Mean±SD) | Amplitude of the pupil dilatation in mm (Mean±SD) | Time taken for full dilatation from instillation of drops in minutes (Mean±SD) | Time taken for full dilation from onset of dilation in minutes (Mean±SD) | Rate of dilatation mm/minute (Mean±SD) |
|---|
| 32 † | 5.30±0.86 | 11.13±5.72 | 2.38±0.76 | 37.44±4.10 | 26.31±7.41 | 0.06±0.02 |
| 94 † | 5.29±0.85 | 8.30±4.92 | 2.39±0.65 | 31.51±4.50 | 23.21±6.85 | 0.08±0.02 |
| p-value of the mean difference of the above two subgroups | 0.962 | 0.008 | 0.948 | <0.05 | 0.032 | 0.003 |
| 126 | 5.29±0.86 | 9.02±5.26 | 2.39±0.68 | 33.02±5.09 | 24.00±7.10 | 0.07±0.02 |
* SD: Standard Deviation, mm: Millimetre, † 32 Patients with reading within 0.1 mm and 94 patients with three similar consecutive readings)
Discussion
Pupil dilatation is important in clinical evaluation and treating posterior segment pathologies as well as for cataract surgeries. Knowing the time it takes for full dilatation will serve as a clinical guide to better organize the clinicians’ and patients’ time. The common drugs used for pupil dilatation are Tropicamide, Tropicamide-Phenylephrine combination and Cyclopentolate. In this study, we used a combination of Tropicamide-Phenylephrine, which is the most common drug in clinical practice for dilating pupils, as its action is faster compared to any other mydriatic [4,5].
Baseline PD can vary with the amount of ambient luminance which might give a false reading of the diameter. An ideal measure would be an instrument which can measure without throwing any light into the pupil. Measurement with a slit-lamp or a ruler with a torch light would give a smaller diameter due to the stimulation and therefore constriction of the pupil. In instruments employing Infra-red source like the OB, there is only minimal light and thus a negligible constriction of the pupil. This is probably the reason why current study showed a larger PD compared to other studies [2,8,9]. To the best of our knowledge, no studies have used OB for measuring the pupil size.
On comparing the values of this study with that of Jethani J et al., [2]; the mean time taken for full dilatation of 34.6±10.5 min is similar, which was 33.02±5.09 min. The baseline PD was 2.1±0.4 mm [2], 1.7±0.5 mm [5], 6.37±0.89 mm in mesopic condition [9] and 5.3±0.8 as studied previously. This difference could be due to a variety of factors from intensity of the ambient illumination to racial differences and stress levels of patients. The amplitude of dilatation in the study done by Yang Y et al., was 4.6 mm in spite of comparable baseline PDs and is probably a reflection of the poor maximal dilatation possible in the Indian population compared to Georgian population [9].
The time of onset of dilatation, rate of dilatation and the time taken for dilatation among the various subgroups, it was seen that patients with larger baseline PD though took more time to start dilating, but reached maximal dilatation earlier than the ones with smaller PD [Table/Fig-3]. A probable reason for the late start could be the smaller surface areas of the iris exposed to the dilating drops compared to the eyes with more constricted pupils. Larger pupils have a shorter distance to reach full dilatation and could be the reason for the shorter time taken for dilation. This trend has not been reported in literature to the best of our knowledge.
The inverse correlation between baseline PD and rate of dilatation could again be due to the smaller amounts of drug in contact with the iris. The time taken to full dilation was the same across the groups because the lesser distance that needed to be travelled by the pupil in dilated eyes compensated for the rate. To the best of our knowledge, there is no study which has measured the rate of dilatation of pupil in any population.
This pupillary dilatation will be adequate for a normal fundus evaluation or for a posterior segment surgery. Hence, a pharmacological acting time of 30 min on an average would be enough in most situations. However, one must remember that around 2% of the patients will take more than 45 min for their complete dilatation. It is possible one drop of Tropicamide-Phenylephrine may not be enough for these patients.
We did not study the participants who had diabetes or other factors that could affect the dilatation of the pupil. Looking at the average values of PD at each time stop of the study, the linear pattern of the pupil dilatation was evident. This also gives us a guide to look at the possibility of screening these patients early after the pharmacological dilatation. One must remember that these pupillary diameters are in patients who are continuing to constrict and not reached maximal dilatation. Thus it is likely that bright light of the slit lamp or the indirect ophthalmoscope would lead to pupil constriction making the examination more difficult. Though the study was not designed to study the natural history of pupil dilatation, it seems that pupil constriction stopped before complete dilatation of the pupil as evidenced from the fact that all patients with fixed dilated pupil and variable pupil readings showed no clinically recognizable constriction on shining a bright light on.
An inverse association was seen between the time taken for dilatation and the baseline PD. A positive correlation between time of onset of dilatation and the baseline PD was seen and the rate of pupil dilatation in Indian population was 0.0735 mm/min in this study population.
Limitation
The error of the OB was not known as the calibration could not be done every day. A significant number of patients in any OPD clinic of Ophthalmology are above 50 years who were not studied and this to a certain extent limits the clinical applicability.
Conclusion
Tropicamide (0.8%) and Phenylephrine (2.5%) combination is the most common dilating agent in clinical ophthalmological practice was effective and provides adequate PD for all clinical situations. Most patients achieved maximal pupil dilatation and a waiting time of 30 minute would be enough in most clinical situations, after the instillation of the pharmacological drop. Baseline PD has an inverse association to the time taken for dilatation and a positive correlation to the time of onset of dilatation.
* SD: Standard Deviation, mm: Millimetre
* SD: Standard Deviation, mm: Millimetre, † 32 Patients with reading within 0.1 mm and 94 patients with three similar consecutive readings)