In modern dentistry, as the patient’s demand for aesthetic dentistry increases, the use of tooth-coloured restorative material increased correspondingly. One among them is the use of PFM restorations. Originally gold alloys were used to make crowns, but with the rise in the price of gold, the search for low cost alloys was initiated. Hence base metal alloys like Ni and Cr based super alloys were introduced by Haynes International, Indiana. The coping used for PFM crowns also shifted from gold alloys to base metal alloys for the same reason. It contains several favourable mechanical properties including high flexural strength, high compression resistance, aesthetic appearance as well as wear resistance, which enables the restoration to mimic the colour, translucency and reflective nature of the natural tooth. In addition, it is biocompatible with coefficient of thermal expansion similar to that of the tooth structure with low thermal and electrical conductance. Thus, this material provides an excellent restorative service as either all ceramic or as PFM restorations. However, fracture of PFM crown is a common problem in restorative dentistry with a prevalence range of about 2.3%-8% [1]. Management of fractured porcelain poses an aesthetic and functional dilemma both for the patient and the dentist. Replacement of failed restoration is not a most practical solution because of economic reason and complex nature of the restoration. This problem has raised the demands for development of practical repair options, which do not necessitate the removal or remake of the entire restoration. Fracture of porcelain in the form of debonding may occur for numerous reasons such as poor metal framework design, faulty technique in fabrication of the porcelain, trauma, occlusal impact, fatigue, micro defects within the material, contamination, parafunctional habits, incompatibility of thermal expansion of coefficient between ceramic and metal or inadequate preparation [2,3]. Various methods have been advocated to repair fractured porcelain with composite resin. One of the major problems in repairing porcelain is bonding the repair composite to fractured surface. The bond strength of composite is also influenced by the bonding agents and the type of composite resin used for repair. Earlier, failed or fractured prosthesis was mostly replaced by a new one. However, this procedure is expensive, demanding and time consuming. With the advent of resin technology, repairing ceramic based failed restorations offer both dentist and patient a cost effective alternative approach, which in turn can increase the clinical longevity of the prosthesis.
Surface pretreatment of fractured ceramic prosthesis is indicated to produce microporosities to increase the surface area, which will enhance the bonding of resin composite and its bond strength at the interface. Numerous techniques have been proposed for this strategy such as, air abrasion with aluminum oxide particle; etching with phosphoric acid, Hydrofluoric Acid (HFA) or Acidulated Phosphate Fluoride (APF) gel; laser irradiation, or combination of these methods [4,5]. The repair technique includes surface preparation and silane treatment in the bonding procedure. Use of lasers for dental applications has increased rapidly since its invention in 1960. Recently, various types of lasers have been suggested for porcelain surface treatment. The newer generation adhesive systems are multipurpose systems capable of bonding composite to enamel, dentin, metal and porcelain. These new adhesive systems can be used for intraoral repair of fractured porcelain restoration by bonding composite [6]. The chemistry of newer systems varies from one manufacturer to other. Hence, present study was conducted to evaluate the efficiency of the two different bonding systems in reference to three different surface treatments on nickel chromium alloys by evaluating the shear bond strength at metal and resin interface.
Materials and Methods
This in vitro study was carried out over a period of six months. A total of 80 Ni-Cr alloys measuring 1×1 cm square blocks were cast using lost wax technique. After casting, the specimens were grouped into four groups based on the surface treatments planned. The four groups were further subdivided into two subgroups based on the bonding technique. The subgroup A samples was treated with SBU, which is a single bottle unique dental adhesive solution that can bond to all surface and subgroup B samples was treated with AP and SBU. AP is dental metal primer, which is used to create a strong bond between the acrylic resin and dental alloys.
Group 1-Neodymium-doped Yttrium Aluminium Garnet (Nd:YAG) laser (n=20);
Group 2-Erbium-doped Yttrium Aluminium Garnet (Er:YAG) laser (n=20);
Group 3-Air abrasion (n=20);
Group 4-No surface treatment (n=20, control group).
All Specimens were embedded into an acrylic mould and fixed with auto polymerised cold cure acrylic resin.
Surface Treatment
Nd:YAG laser: The Ni-Cr metal alloy (n=20) surfaces were irradiated by linear movement of a glass fibre of Nd:YAG lasers at a power setting of 6 W, representing energy and frequency levels at a depth of 300 μ in 2 mm space interval. The specimens were then cleaned in an ultrasonic bath for 10 minutes. The samples were then randomly divided into two subgroups based on the bonding technique as SBU (subgroup-1a) and AP+SBU (subgroup-1b).
Er:YAG laser: Similarly the 20 metal surfaces were irradiated by a non-contact headpiece (RO2) of Er: YAG at a power setting of 6 W, representing energy and frequency levels of 200 mJ with 30 Hz frequency all over the metal surface. After ultrasonic cleansing, the samples were sub-grouped as above SBU (subgroup-2a) and AP+SBU (subgroup-2b).
Air abrasion procedure: After embedding the alloys (n=20) in acrylic block, it was ultrasonically cleaned. Then, sand blasting was done using 120 μm alumina particles. The distance between the jet nozzle and the sample was maintained at 5 cm. Blasting was performed at 0.4 MPa for 30 seconds. The samples were then rinsed in an ultrasonic bath for 10 minutes to remove any surface impurities with acetone [5]. The samples were then randomly divided into two subgroups based on the bonding technique as SBU (subgroup-3a) and SBU+AP (subgroup-3b). The samples of control group was divided in two subgroups and treated as above without any surface treatment (subgroup-4a,4b).
Application of Bonding Agent
Single bond universal: The alloy surfaces of all samples in subgroup A were coated with SBU (3M, ESPE) as per manufacturer’s instructions. After drying the alloy surfaces, a drop of bonding agent is dispensed and applied over the metal for 20 sec using a disposable applicator. A new applicator tip was used for each specimen. Subsequently, a gentle stream of air is directed over the liquid for about 5 seconds until the solvent has evaporated completely. The bonding agent is then cured for 10 seconds using a routine curing light.
Alloy primer: For all samples in subgroup B, AP (Kuraray Medica Inc. Japan) were applied to the Ni-Cr alloy surface with a disposable applicator tip for 20 seconds and left to dry for 60 seconds at room temperature. Once the primer is set leaving a frosty appearance on the alloy surface the specimens were treated with SBU same as the above SBU group.
After surface treatment of the sample, a clear plastic tube measuring 4 mm diameter and 4 mm length was placed over the samples and composite resin, Filtek™ Z 350 (3M™ ESPE™), which is an universal nanofill composite of shade A3D was condensed inside the plastic tube and light cured for 60 seconds with XL 3000 light curing unit (3M ESPE), under standard irradiation mode at 650 mW/cm2. The samples were then stored at 37°C for 24 hours to simulate mouth temperature before mechanical testing. Shear testing of all groups was performed on Universal testing machine (Instron 3382) using a cross head speed of 1 mm/min. The chisel was positioned at the interface between the Ni-Cr surface and the composite and perpendicular load was applied at the metal resin interface. The shear debonding forces were recorded in kilograms and converted into MPa.
Statistical Analysis
All statistical analysis was performed using the Statistical Package for Social Sciences, vers.10.0 (SPSS, Chicago, IL). The means and standard deviations of SBS values in MPa were calculated for each group, and the results were statistically analysed with one-way ANOVA and Tukey HSD tests. Statistical analysis by ANOVA reveals a statistically significant difference when bond strength values were compared among the groups (p<0.05).
Results
Shear bond strength values were highest in group 2b (31.23±5.18) followed by 2a (26±4.64) and it was low in group 3a (17.74±2.11) [Table/Fig-1]. The mean bond strengths, standard deviations, and group differences for the six different surface treatment groups are shown in [Table/Fig-2]. Post Hoc analysis reveals that air abrasion and laser groups have a statistically significantly difference. No statistically significant difference was seen in between SBU and AP in both air abrasion and laser pretreatment. In the study groups, the lowest bond strength was observed for the air abrasion (18 MPa). No statistically significant difference was observed between the Er:YAG subgroups and these groups demonstrated higher bond strengths when compared with the Nd:YAG laser group (p<0.05). The control groups demonstrated a statistically significantly lower bond strength value (9 MPa and 11 MPa respectively).
Comparison of shear bond strength.
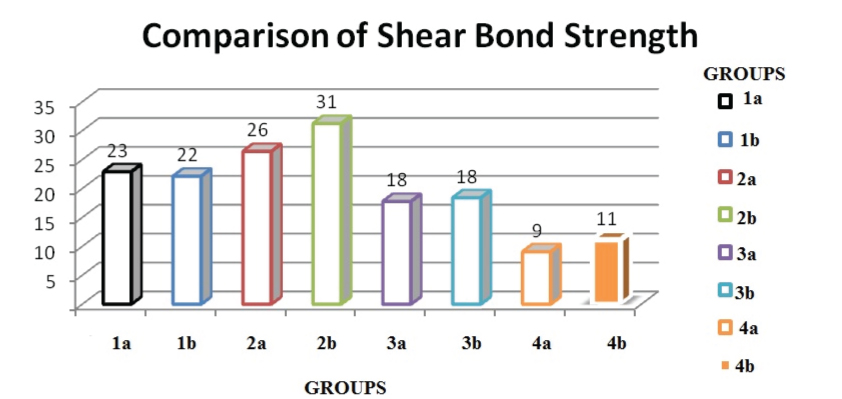
Mean and standard deviation of shear bond strength values in MPa.
| Groups | N | Mean (Mpa) | Standard deviation | Standard error | Minimum | Maximum |
|---|
| Group 1a | 10 | 22.881 | 3.592 | 1.136 | 17.89 | 28.68 |
| Group 1b | 10 | 22.108 | 4.393 | 1.389 | 14.72 | 29.11 |
| Group 2a | 10 | 26.402 | 4.648 | 1.469 | 19.32 | 32.72 |
| Group 2b | 10 | 31.232 | 5.181 | 1.638 | 24.36 | 39.37 |
| Group 3a | 10 | 17.749 | 2.111 | 0.667 | 14.37 | 20.42 |
| Group 3b | 10 | 18.389 | 3.292 | 1.041 | 14.58 | 22.53 |
| Group 4a | 10 | 9.059 | 2.515 | 0.795 | 6.51 | 14.06 |
| Group 4b | 10 | 11.132 | 2.571 | 0.813 | 8.65 | 17.62 |
Discussion
For decades, metal ceramic dental prostheses have been used in dentistry with good clinical performance, aesthetics and durability. The aim of these restorations is to combine the fracture resistance of the metal substructure with the aesthetic properties of porcelain. Noble alloys have always been the first choice, mainly those containing gold, palladium and platinum. However, due to economic reasons, the use of noble alloy is diminishing in the day-to-day use. This led to the widespread use of base metal alloys, such as Ni-Cr or Cobalt-Chromium (Co-Cr), as frameworks for porcelain, due to their high mechanical strength, high modulus of elasticity and good adhesion to porcelain [7].
Fracture of a porcelain restoration is often considered as an emergency treatment and represents a challenge for the dentist. Over the last few years, various surface pretreatment In vitro studies have been carried out to evaluate bond strength at resin metal interface. Currently, newer chemical adhesion systems have been introduced containing acid monomer, which enable better adhesion both to metal, resin or enamel [8,9]. Agents such as cyanoacrylate, acrylic resin have been used to repair metal ceramic restoration with limited success. Composite resin has been the material of choice for their ease of manipulation and aesthetic value [10]. Intraoral repair of porcelain fractures in PFM restorations has been a point of consideration; since, replacement of these restorations does not seem to be economic and in some cases not practical. Various methods have been introduced to repair fractured porcelain using a composite resin. Mechanical roughening of the surface with a coarse diamond, air abrasion (sandblasting) with aluminum oxide, etching with HFA or phosphoric acid have been done to facilitate micromechanical retention [11,12].
In present study, the effect of three surface treatments viz., air abrasion and laser etching using Er:YAG and Nd:YAG were evaluated. The specimens were divided into two subgroups (A and B). Subgroup A was treated with the bonding agent alone (SBU) and Subgroup B was treated in addition with AP.
Air-borne abrasion is a conventional treatment modality, which produce uniform surface roughness. It is often used to clean the surfaces of the materials and is used to achieve a microretentive topography and to increase surface area of restoration. The abrasion of alumina was performed to mechanically clean the surface and to increase the bonding surface area thereby increasing the surface energy [13]. It was proved alumina particle when used for sand blasting, there are complex reactions taking place like separation and accumulation of certain elements at the surface; thereby contaminating it. In present study, air abrasion group showed least shear bond strength [13]. However, sandblasting the metal surface especially with alumina has the potential to remove the clinical adaptation of the resin composite [14]. Thus, unnecessary sandblasting should be avoided as it is likely to cause more damage.
Considering the current advances in laser technology, laser beam irradiation is used for surface treatment. When compared among the laser etched groups, the group that was surface treated with Er:YAG laser group showed higher shear bond strength when compared to Nd:YAG laser. The principal effect of laser energy is the conversion of light energy into heat, and the most important is interaction between the laser energy and the substrate, Er:YAG laser is one of the most promising laser types for this purpose [15,16]. It has been attributed that laser etching increases surface roughness of the base metal alloys, which indeed allowed for greater micromechanical bonding. The higher bond strength of Er:YAG laser could be due to increased surface roughness without any microcracks formation [17]. Previous studies have shown that Er:YAG produces a highly rough surface which in turn resulted in high bond strength values. Thus, the surface microroughness facilitates the penetration of resin tags and cement into such irregularities produced by laser and improve adhesion [18,19]. Due to its good interaction with dental structures, Er:YAG laser can be a favourable alternative for repair procedures on ceramic materials.
When compared among the subgroups, the sample that was treated with AP (subgroup b) showed higher bond strength and was statistically significant. AP is a metal conditioning agent used to enhance the bond strength between dental metals and resin based materials [20]. This could be attributed to the presence of 10-Methacryloyloxydecyl Dihydrogen Phosphate (MDP), which react chemically with the chromium oxide layer created on the cast metal surfaces by means of covalent bonds. MDP has an ester phosphate group that presents great chemical bonding with the surface layer of oxide of chrome formed in the surface of the alloy, which can be highly reliable to promote better union [20-22]. In addition, the AP can be used both intra and extra-orally due to its clinical versatility. It enhances the bond strength to both precious and also non-precious metals.
Limitation
In vitro analysis is the first step of testing any technique or material to examine their properties and its potential. The limitation of the present study is that it tested only the SBS among the groups and in future, it is suggested that other aspects of the bond (type of failures, different mechanical test design, microleakage and thermocycling) need to be studied for more comprehensive evaluation. A successful outcome for any bonding depends on the proper technique and complete knowledge on the materials used. Selecting the appropriate boding system plays a vital role in achieving highest shear bond strength with standardised surface pre-treatment.
Conclusion
Within the limitations of this in vitro study, it was concluded that Er-YAG laser treated alloy surfaces bonded along with AP displayed highest shear bond strength. Subgroup B samples bond strength was significantly higher. Both mechanical (laser) and chemical {10-MDP and VBATDT (6-(4-vinylbenzyl-n-propyl) amino-1,3,5-triazine-2,4 dithiol)} factors in this group contributed to high bond strength.