Pulmonary tuberculosis is one of the major chronic infectious disease and major cause for morbidity and mortality to the mankind. Globally, about 6.3 million new cases of TB were reported in 2016 and worldwide it is the 9th leading cause of death [1]. In India, TB is one of the major public health burden and accounts for one fourth of global TB burden [2].
The inflammation is an immunological defence mechanism of the body when it meets challenges like injury, infection and allergy which results in the significant immigration of White Blood Cells (WBCs) and release of cytokines [3]. The TNF-α is one of the major inflammatory cytokine secreted from macrophages, dendritic cells and T cells during infectious disease like PTB which is essential for host defense against Mycobacterium tuberculosis and also promotes granuloma formation [4]. Perhaps TNF-α is an indispensible inflammatory cytokine with multiple roles in both immunological and pathophysiological responses in TB [5].
EPO is an endogenous glycoprotein hormone produced mainly by kidney and is crucial for controlling Red Blood Cell (RBC) production. It stimulates erythropoiesis by acting on the bone marrow where it promotes terminal differentiation of progenitor cells into erythrocytes [6]. Indeed the potent stimulus for increased production of EPO is diminished arterial oxygen content along with anaemia or hypoxia [7].
In chronic diseases like PTB, anaemia is the most common haematological disorder with multifactorial aetiology, however inflammatory cytokine and iron deficiency have been indicated in the pathophysiology of anaemia in PTB [8,9]. In the presence of increased levels of TNF-α, the production of EPO is impaired and the biological activity is reduced [10].
Probably, to know the disease severity, extent of disease, prognosis and monitoring the clinical effect of Anti Tuberculoses Therapy (ATT), estimation of immunological and pathological parameters seems to be the better predictive factors in PTB. From this background, the present study was carried out with the hypothesis that increased bacterial burden increases TNF-α level which in turn influences EPO action on pathophysiology of anaemia during chronic infection like PTB.
Materials and Methods
Study Design and Population
The present cross-sectional study was conducted at Navodaya Medical College Hospital and Research Centre, Raichur, Karnataka, India from January 2016 to January 2017. The study included 180 newly diagnosed sputum positive PTB patients as cases. The clinical diagnosis of cases was done by pulmonologist and PTB was confirmed by microscopic examination of sputum specimen for the detection of AFB. The age and sex matched 100 healthy individuals were included as controls. The study protocol was approved by Institutional Human Ethics Committee (IEC Ref No.-59/2015-16). Before enrolment, written informed consent was taken from all the study participants after explaining in detail about study procedure.
Inclusion criteria: The subjects diagnosed as “new cases” of PTB, possessing at least two sputum smear test positive for AFB were included as cases and the healthy individuals with no previous history of any major diseases were included as controls in the present study.
Exclusion criteria: The patients with extrapulmonary TB and/or patients requiring surgical intervention, patients with history of prior anti TB treatment, patients with other lung disorders, patients with HIV, with organ transplantation, treatment with corticosteroids, with chronic renal failure, diabetes mellitus, liver failure and patient with recent myocardial infarction were excluded from the study.
Haematological and Biochemical Parameters
About 6 mL of venous blood sample was drawn from median cubital vein under aseptic condition from each study subjects. Out of which, one part of blood sample (2 mL) was collected in an EDTA vial and immediately processed for haematological parameters using automated cell counter (ABX Pentra 60, Horiba, Japan) by flow cytometry method.
The second part of blood sample collected in plain vial were allowed to clot for 30 minutes and then centrifuged at 3000 rpm for 10 minutes. The separated serum samples were stored at -80°C until assayed. Serum levels of TNF-α and EPO in all the study participants were measured by sandwich ELISA method. Serum levels of TNF-α were measured by using commercially available TNF-α ELISA kit (Diaclone, France) according to manufacturers’ instructions [11]. Serum EPO was estimated by using commercially available EPO ELISA kit (Biomerica, USA) according to manufacturers’ instructions [12].
Statistical Analysis
The statistical analysis was performed by using SPSS software version 16.0. The results were analysed with descriptive statistics, wherever appropriate. The Student unpaired t-test, and Pearson’s correlation coefficient test were used to evaluate the statistical significance in the results. We compared mean values for serum TNF-α, EPO and Hb levels in PTB patients with different sputum AFB grading using Kruskal-Wallis test [13]. The p-value of <0.05 was considered as threshold of significance.
Results
In the present study, 180 newly diagnosed sputum positive PTB cases with mean age of 44.1±16.2 years (age range 19-75 years) and 100 healthy controls with mean age of 43.49±15.6 years (age range 19-72 years) were included. Among 180 PTB cases 101 were males and 79 were females and in 100 controls 54 were males and 46 were females. There was no difference between cases and controls with respect to age and sex.
The mean level of Hb, RBC, Packed Cell Volume (PCV), Mean Corpuscular Volume (MCV), Mean Corpuscular Haemoglobin (MCH) and Mean Corpuscular Haemoglobin Concentration (MCHC) were lower while WBC, platelets, Erythrocyte Sedimentation Rate (ESR), TNF-α and EPO were found to be higher in PTB cases compared to healthy controls (p<0.05) as illustrated in [Table/Fig-1].
Comparison between mean of different parameters in PTB cases and healthy controls.
| Parameters | Healthy control (Mean±SD) | PTB cases (Mean±SD) | p-value |
|---|
| Hb (gm/dlL) | 14.10±1.21 | 10.30±2.0 | <0.001* |
| RBC (Millions/cumm) | 4.96±0.57 | 4.05±0.75 | <0.001* |
| PCV (%) | 41.50±3.40 | 30.53±5.79 | <0.001* |
| MCV (fL) | 85.50±7.47 | 75.45±9.73 | <0.001* |
| MCH (pg/cell) | 29.20±2.01 | 25.51±3.90 | <0.001* |
| MCHC (gm/dL) | 34.10±1.21 | 33.65±2.49 | <0.05* |
| WBC (Cells/cumm) | 6574±1360 | 10227±3645 | <0.001* |
| Platelets (Lakhs/cumm) | 2.96±0.70 | 4.24±1.42 | <0.001* |
| ESR (mm/hour) | 5.83±3.34 | 67.40±22.10 | <0.001* |
| TNF-α (pg/mL) | 14.40±3.72 | 90.68±31.69 | <0.001* |
| EPO (mIU/mL) | 17.30±6.60 | 35.87±7.82 | <0.001* |
*p<0.05 is significant
PTB: Pulmonary tuberculosis; Hb: Haemoglobin; RBC: Red blood cell; PCV: Packed cell volume; MCV: Mean corpuscular volume; MCH: Mean corpuscular haemoglobin; MCHC: Mean corpuscular haemoglobin concentration; WBC: White blood cell; ESR: Erythrocyte sedimentation rate; TNF-α: Tumour necrosis factor-alpha; EPO: Erythropoietin
We noted increased serum levels of TNF-α and EPO in PTB cases when compared to controls (p<0.001 and p<0.001 respectively) as shown in [Table/Fig-1]. Further, we compared mean values for TNF-α, EPO and Hb levels in PTB cases with different sputum AFB gradings. Chi-square test showed statistically significant associations between TNF-α, EPO and Hb with bacterial burden (p<0.001) measured in terms of sputum AFB gradings in PTB [Table/Fig-2].
Comparison between mean±sd values for TNF-α, EPO and Hb in PTB cases with different sputum AFB grading.
| Sputum AFB grading | TNF-α (pg/mL) | EPO (mIU/mL) | Hb (gm/dL) |
|---|
| Scanty (n=14) | 33.18±5.73 | 32.81±6.18 | 12.81±1.2 |
| 1+ (n=41) | 52.74±16.24 | 40.53±7.15 | 10.97±1.55 |
| 2+ (n=41) | 84.14±20.56 | 37.40±7.0 | 9.84±1.4 |
| 3+ (n=73) | 112.45±28.46 | 33.73±6.20 | 8.90±1.2 |
| 4+ (n=11) | 177.0±17.94 | 24.45±6.30 | 7.21±0.7 |
| Chi square value | 126.46 | 51.06 | 71.34 |
| p-value | <0.001* | <0.001* | <0.001* |
*p<0.05 is significant by Kruskal-Wallis test
AFB: Acid fast bacilli; n: Number of pulmonary tuberculosis cases; TNF-α: Tumour necrosis factor-alpha; EPO: Erythropoietin; Hb: Haemoglobin; PTB: Pulmonary tuberculosis
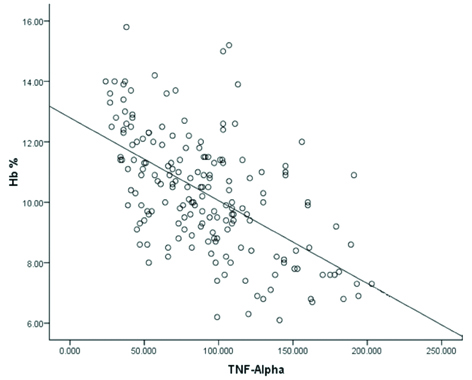
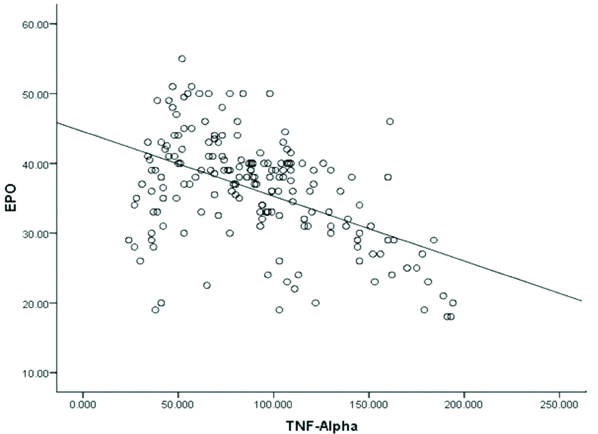
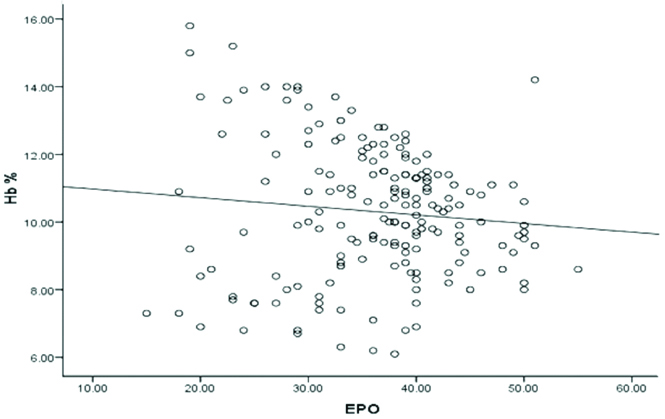
We observed statistically significant inverse correlation between serum TNF-α and Hb level (r=-0.58, p<0.001) in PTB cases [Table/Fig-3] and also between serum levels of TNF-α and EPO (r=-0.49, p<0.001) in PTB cases [Table/Fig-4]. Furthermore, we found statistically insignificant correlation between serum EPO and Hb level (r=-0.101, p>0.05) in PTB cases [Table/Fig-5].
Correlation between serum TNF-α (pg/mL) and Hb (gm%) level in PTB patients (r=-0.58, p<0.001).
p<0.05 is significant
TNF-α: Tumour necrosis factor-alpha; Hb: Haemoglobin; PTB: Pulmonary tuberculosis
Correlation between serum TNF-α (pg/mL) and EPO (mIU/mL) levels in PTB patients(r=-0.49, p<0.001).
p<0.05 is significant
TNF-α: Tumour necrosis factor-alpha, EPO: Erythropoietin; PTB: Pulmonary tuberculosis
Correlation between serum EPO (mIU/mL) and Hb (gm%) level in PTB patients (r=-0.101, p>0.05).
p>0.05 is not significant
EPO: Erythropoietin; Hb: Haemoglobin; PTB: Pulmonary tuberculosis
Discussion
In the present study, we aimed to estimate the role of TNF-α on EPO function and its correlation with haematological profile in different grades of PTB. The abnormal haematological parameters in PTB patients observed in the present study were in accordance with previous studies [12,14-18]. Present study result showed statistically significant differences in serum levels of TNF-α and EPO between PTB cases and controls. We observed inverse correlation between TNF-α and EPO and between TNF-α and Hb in PTB. Further, we also noticed statistically insignificant correlation between EPO and Hb in PTB cases.
In PTB the invading bacteria induces the synthesis of TNF-α along with other inflammatory cytokines through the activation of monocytes and T-lymphocytes. There was a significant positive correlation between clinical severity and serum TNF-α level. Further to monitor the disease severity, TNF-α seems to be a useful marker in PTB patients [19]. Similarly in the present study, we have found increased serum TNF-α level in PTB patients with increase in bacterial load. The subnormal Hb associated with severity of PTB as observed in present study was considered to be multifactorial and might be related to the underlying inflammatory mechanism.
Few studies have reported blunted EPO response in the presence of increased TNF-α level in chronic disorders [12,20-23]. The documentary evidence of these studies throws more light on the fact that in kidney TNF-α inhibits EPO production and interferes with EPO mediated expression of specific transcription factors involved in erythrocyte differentiation control [24,25]. Further, the TNF-α directly inhibits the proliferation and differentiation of erythroid progenitor cells. In addition, TNF-α along with IL-1, IL-6 and IL-10 as a defence against invading microbial pathogens to effectively withhold the iron from microbes, induces the expression of ferritin and stimulates iron storage and retention within the macrophage. The resulted low circulatory iron fortuitously decreases the iron supply for erythroid cells establishing hypoferremia. Thus, ineffective erythropoiesis together with relative inadequacy of EPO production contributed in low Hb and subsequent development of anaemia of chronic disease [20,24,26].
The results of present study probably explained on the basis of above findings, wherein we have reported, commensurate increase in serum TNF-α and decrease in Hb level with increased bacterial burden in PTB patients. Despite of increased serum EPO in PTB cases compared to controls, present study results strongly indicated a relative lack of endogenous EPO in proportion to the Hb concentration. The EPO down regulation in present study might be due to the burst of inflammation that took place during increased bacterial burden with concomitant increase of TNF-α which might have ultimately contributed in the development of anaemia in PTB.
Few studies have highlighted on increased levels of serum EPO in both anaemia of chronic disease like PTB and iron deficiency anaemia however, found more significant levels of EPO in iron deficient subjects indicating a role of inflammatory cytokines on EPO response in chronic diseases [18,21]. Yet another study demonstrated comparatively low levels of serum EPO in patients with chronic infection and malignancy than in patients with iron deficiency anaemia and primary haematopoietic disease with same degree of anaemia [27]. The TNF-α mediated reduction of both EPO as well as erythropoietin receptor protein advancing to ineffective erythropoiesis in β-Thalassaemia/haemoglobin E patients has been also reported by another researcher [28].
Kulkarni RA et al., compared the levels of TNF-α, EPO and Hb in PTB patients and found inverse correlation between TNF-α and Hb and between TNF-α and EPO. Further in the same study, they found no inverse correlation between EPO and Hb however, blunted response of EPO to the degree of anaemia was reported [11]. However, a similar type of study conducted recently on COPD patients reported an inverse correlation between serum EPO level and both Hb and haematocrit levels [23].
Concurrent findings were observed in present study with significant inverse correlation between TNF-α and Hb levels and also between TNF-α and EPO levels in PTB cases. Furthermore, the expected inverse correlation between serum EPO and Hb levels was absent in PTB cases. These findings showed that TNF-α along with other cytokines might inhibit EPO production and also induce the formation of nitric oxide and oxygen free radicals which directly inhibit the expression of EPO and damage EPO producing cells [29]. In addition, these cytokines alters iron metabolism, inhibits proliferation of erythrocyte progenitor cells and also reduces EPO receptor proteins leading to EPO resistance and resultant under maintenance of Hb level [28,30]. Further in continuation to this, we have grouped the PTB patients according to sputum AFB gradings to show the disease severity and compared the mean values for serum TNF-α, EPO and Hb levels which were not done previously. The [Table/Fig-2] clearly showed that, increased bacterial burden measured in terms of sputum AFB grade resulted in increased activity of TNF-α in PTB cases. This shows that there was a direct association between bacterial burden and serum TNF-α. There was a decreasing trend among serum EPO and Hb levels with agreement with severity of PTB. Initially, when the disease severity were less, we found raised serum EPO level in PTB cases which could be due to little increase in serum TNF-α at that time. However, as the bacterial burden increased, we observed down fall of both serum EPO and Hb levels, which might be due to the inhibitory effect of increased TNF-α in PTB cases. The PTB is most commonly seen in people with low socioeconomic and poor nutritional status and hence anaemia of inflammation as well as iron deficiency anaemia may occur simultaneously in PTB patients. Probably in the present study, absence of coordination between EPO and Hb due to increased expression of inflammatory cytokine TNF-α might have progressed towards the establishment of anaemia in PTB cases.
One of the stimulus for increased production of EPO is low Hb level, however in the present study, none of the healthy controls showed low levels of Hb and hence there was no increase in serum EPO in controls [31]. However, many PTB cases of the present study showed low levels of Hb. This might have stimulated EPO production in the initial stages, when disease severity was less as evident from sputum AFB gradings and little increase in serum TNF-α. Therefore, there was no significant inhibitory effect of TNF-α on EPO. Probably this was the reason behind higher EPO in PTB cases than controls as observed in present study. Further it is stated that, as the disease severity was increased, despite of low Hb, the level of EPO could not increase which might be due to the suppression of EPO by increased levels of TNF-α and further anaemia in PTB. Thus, in the present study, so far we were able to show the link between Hb concentration and serum levels of both TNF-α and EPO in PTB patients with different sputum AFB gradings.
Limitation
Further extended large scale study would be required to attain firm conclusion. In the present study, we have not done follow-up of PTB patients which could have been more informative in evaluating prognostic applications of inflammatory marker and EPO.
Conclusion
The present study highlighted on the fact that increased bacterial burden was associated with increased serum TNF-α level. The relative deficiency of serum EPO levels in proportion to Hb concentration which played a vital role in the pathophysiology of anaemia was due to the increased TNF-α in PTB. Thus, we concluded that during treatment, to reduce the negative prognostic impact of anaemia associated with reduced survival and increased morbidity, we suggest therapeutic administration of recombinant human EPO could be helpful to overcome anaemia in PTB.
*p<0.05 is significant
PTB: Pulmonary tuberculosis; Hb: Haemoglobin; RBC: Red blood cell; PCV: Packed cell volume; MCV: Mean corpuscular volume; MCH: Mean corpuscular haemoglobin; MCHC: Mean corpuscular haemoglobin concentration; WBC: White blood cell; ESR: Erythrocyte sedimentation rate; TNF-α: Tumour necrosis factor-alpha; EPO: Erythropoietin
*p<0.05 is significant by Kruskal-Wallis test
AFB: Acid fast bacilli; n: Number of pulmonary tuberculosis cases; TNF-α: Tumour necrosis factor-alpha; EPO: Erythropoietin; Hb: Haemoglobin; PTB: Pulmonary tuberculosis