Effect of Magnesium Sulphate and Dexmedetomidine on Blood Loss during Lumbar Spinal Fusion Surgeries
Titu George Oommen1, Sivakumar Segaran2, Mamie Zachariah3, RV Ranjan4, Anil Radhakrishnan Pillai5, Sunilkumar Valasareddy6, Nagalakshmi7
1 Senior Resident, Department of Anaesthesia, Pondicherry Institute of Medical Sciences, Pondicherry, India.
2 Assistant Professor, Department of Anaesthesia, Pondicherry Institute of Medical Sciences, Pondicherry, India.
3 Professor, Department of Anaesthesia, Pondicherry Institute of Medical Sciences, Pondicherry, India.
4 Professor, Department of Anaesthesia, Pondicherry Institute of Medical Sciences, Pondicherry, India.
5 Senior Resident, Department of Anaesthesia, Pondicherry Institute of Medical Sciences, Pondicherry, India.
6 Senior Resident, Department of Anaesthesia, Pondicherry Institute of Medical Sciences, Pondicherry, India.
7 Assistant Professor, Department of Anaesthesia, Pondicherry Institute of Medical Sciences, Pondicherry, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Sivakumar Segaran, Assistant Professor, Department of Anaesthesia, Pondicherry Institute of Medical Sciences, Pondicherry-605014, India.
E-mail: siva85dr@gmail.com
Introduction
Spine surgeries pose unique challenges to the anaesthesiologist in maintaining stable haemodynamics, relatively dry operative field and concerns over patient positioning during surgery.
Aim
The present study was aimed at comparing the effect of magnesium sulphate and Dexmedetomidine in minimizing blood loss and maintaining haemodynamic stability during spinal fusion surgeries.
Materials and Methods
Forty two patients aged 18-60 years of ASA 1 and 2 were randomly divided into two groups A and B. Group A received 30 mg/kg bolus followed by 10 mg/kg/hr of magnesium sulphate and group B received 1mcg/kg bolus followed by 0.4mcg/kg/hr of Dexmedetomidine till the end of surgery. The study drugs were started after intubation and positioning prone. Both the groups were observed for changes in haemodynamic parameters and for amount of blood loss. Blood loss was assessed by weighing the soaked sponges and gauze and calculating against the dry weight. A 1ml of blood was considered to be equal to 1gram weight. Statistical analysis was performed using SPSS version 21.
Results
The mean blood loss in group A was 516±8.029 ml and 499±5.34 mL in group B (p>0.05). The heart rate, systolic blood pressure, diastolic blood pressure and mean arterial pressure were lower in group A compared to group B at various time intervals throughout the study (p<0.05) and it was statistically significant.
Conclusion
Both magnesium sulphate and Dexmedetomidine can be used for minimising blood loss during lumbar spinal fusion surgeries with Dexmedetomidine maintaining better haemodynamic stability.
Anaesthesia, Deformity correction surgeries, Haemodynamic stability
Introduction
Spine surgeries pose unique challenges to the anaesthesiologist in maintaining stable haemodynamics, relatively dry operative field and concerns over patient positioning during surgery. There is increasing number of adult deformity correction surgeries being performed with multiple levels of fusion required; various studies have reported blood loss ranging from 1 to 3 lt for posterior spinal fusion surgeries [1-5]. Perioperative blood loss means increased transfusion requirements which in turn lead to disease transmission or transfusion related reactions. Significant blood loss also results in greater fluid shifts, which can affect cardiac, pulmonary and renal status, or even, in the extreme case, leads to Transfusion-Related Acute Lung Injury (TRALI) [6]. Due to the heightened awareness of the potential deleterious effects of allogeneic blood product administration, several techniques have been evaluated to determine their efficacy in limiting perioperative blood loss. These techniques include autologous transfusion therapy, intraoperative and postoperative blood salvage, pharmacologic manipulation of the coagulation cascade, and controlled hypotension [7]. Controlled hypotension is a well-recognised technique which helps in preventing perioperative blood loss.
The drug used for controlled hypotension must follow certain characteristics, such as ease of administration, a short onset time, an effect that disappears quickly when administration is discontinued, rapid elimination without toxic metabolites, negligible effects on vital organs, and predictable dose-dependent effects [8].
Magnesium Sulphate (MgSO4) is a non-competitive N-Methyl-D-Aspartate (NMDA) receptor antagonist with anti-nociceptive effects [9]. Numerous clinical investigations have demonstrated that magnesium infusion during general anaesthesia reduces anaesthetic requirement and postoperative analgesic consumption [10,11]. In addition, Mg++ inhibits the release of norepinephrine by blocking the N-type Ca++ channels at nerve endings and thus decreases the blood pressure. In high doses, it causes muscle paralysis, vasoplegia, respiratory depression and prolongation of action of muscle relaxants. Dexmedetomidine is a highly selective α2 adrenoceptor agonist with sedative, anxiolytic and analgesic characteristics. Dexmedetomidine mediates central α2A and imidazoline type 1 receptors [12-14]. The activation of these central receptors results in a decrease in norepinephrine release and leads to a decrease in blood pressure and heart rate. It causes bradycardia, hypotension, drowsiness, arrhythmias in susceptible individuals and decreases MAC (Minimum Alveolar Concentration) of volatile anaesthetics. There are several studies which have assessed the effectiveness of Dexmedetomidine and magnesium sulphate in controlled hypotension. These two agents [15-17] have been compared with other agents [18,19] in terms of their role in hypotensive anaesthesia, but to the best of our knowledge, no study comparing these two agents with each other for spine fusion surgery has been cited in the scientific literature.
Hence, the present study was aimed at comparing the effect of these two drugs in minimising blood loss and maintaining haemodynamic stability in lumbar spinal fusion surgeries.
Materials and Methods
This prospective randomised double blinded comparative study was conducted from October 2014 to September 2016 in Pondicherry Institute of Medical Sciences after approval from institution ethical committee (IEC no: RC/14/53, CTRI ref.no:2014/10/007756) and written informed consent from all the patients. A total of 42 patients aged 18 to 60 years, ASA PS (American society of Anaesthesiologists physical status) I or II of either sex and scheduled for elective lumbar spinal fusion surgeries under general anaesthesia were included in the study. Patients with cardiovascular, cerebrovascular and neuromuscular disorders, BMI (Body Mass Index) <18 and >30 kg/m2, chronic renal disease, coagulation abnormalities, patients on beta blockers and calcium channel blockers were excluded from the study. The patients were randomised into 2 groups of 21 each using concealed random numbers method till required sample size was obtained [Table/Fig-1].
Consort Design.
Group A-Magnesium sulphate; Group B-Dexmedetomidine
All selected patients were pre medicated with Tab. Lorazepam 1mg and Tab. Ranitidine 150 mg orally 2 hours before surgery. On arrival to operating room, intravenous line with 16 G cannula and invasive blood pressure monitoring via 20 G cannula in the radial artery were secured under local anaesthesia. The patients were monitored with ECG (Electrocardiogram), IABP (Intra Arterial Blood Pressure Monitoring), pulse oximeter and capnograph after induction of general anaesthesia. All patients were preloaded with 10ml/kg of lactated Ringer solution before induction of anaesthesia.
After preoxygenation, patients were induced with inj. Glycopyrollate 0.2mg, inj. Fentanyl 2 mcg/kg and inj, Thiopentone sodium 5 mg/kg, endotracheal intubation facilitated with inj. Vecuronium 0.1 mg/kg and patients were intubated with appropriate size endotracheal tube. Anaesthesia was maintained using oxygen, nitrous oxide and isoflurane to achieve MAC value of 1. Then patients were positioned prone and pressure points were padded and again tube position was confirmed. After positioning the patient prone, those who were assigned to Group A received magnesium sulphate (30mg/kg loading dose over 10 minutes followed by infusion of 10 mg/kg/hr till the end of surgery) and those assigned to Group B received Dexmedetomidine (1mcg/kg loading dose over 10 minutes followed by infusion of 0.4 mcg/kg/hr till the end of surgery).
Patient’s vital signs were monitored and recorded every 5 minutes. All patients received inj. Paracetamol 1gm i.v. 1 hour after incision. Neuromuscular blockade was maintained with inj. Vecuronium 1mg every 20-30 minutes. The study drug infusion was discontinued when the surgeon started skin closure. At the end of surgery, patients were positioned supine and neuromuscular blockade was reversed with inj. Neostigmine 0.05 mg/kg and inj. Glycopyrollate 0.01mg/kg and patients were extubated when they were fully awake and obeyed commands. Neurological assessment was done by the surgeon and shifted to the recovery room.
Blood loss was assessed by weighing the soaked sponges and gauze and calculating against the dry weight. A 1ml of blood was considered to be equal to 1gram weight. The blood in the suction bottle was measured taking into account the irrigation fluid used. Any drop in MAP (Mean Arterial Pressure) below 55 mmHg was managed with 100 ml fluid boluses, if it still did not respond inj.Ephedrine 6 mg bolus was given and repeated if needed after 3 minutes. If it still does not responds, other vasopressors would be started and study drug would have been stopped. Any incidence of bradycardia {Heart rate (HR) <50 bpm} would be managed with inj.Atrophine 0.4 mg.
Sample size was calculated, assuming to measure a difference of 60 mL of mean blood loss between the 2 groups as significant with 95% confidence interval and type II error 80%. The sample size was calculated to be 40 patients (20 patients/ each group) which was increased to 42 using Epi info. Statistical analysis were performed using SPSS version 21 and Microsoft Excel 2013 was used for data entry. Continuous quantitative data were expressed as mean and standard deviation, median and inter-quartile range. Mann-whitney U test was used for comparison of means for non- parametric data. Independent sample t test was used for comparison of means when the distribution of data was nominal. The p-value of <0.05 was considered significant.
Results
All 42 patients enrolled in the study completed the study. The demographic profile between the two groups were comparable [Table/Fig-2].
| Age (in years) | Dexmedetomidine | Maganesium sulphate | Total | p-value |
|---|
| 19-30 | 1 | 0 | 1 | 0.653 |
| 31-40 | 9 | 7 | 16 |
| 41-50 | 4 | 8 | 12 |
| 51-60 | 5 | 6 | 11 |
| Total | 21 | 21 | 42 |
| Gender | Dexmedetomidine | Maganesium sulphate | Total | p-value |
| Male | 11 | 13 | 24 | 0.756 |
| Female | 10 | 8 | 18 |
| Total | 21 | 21 | 42 |
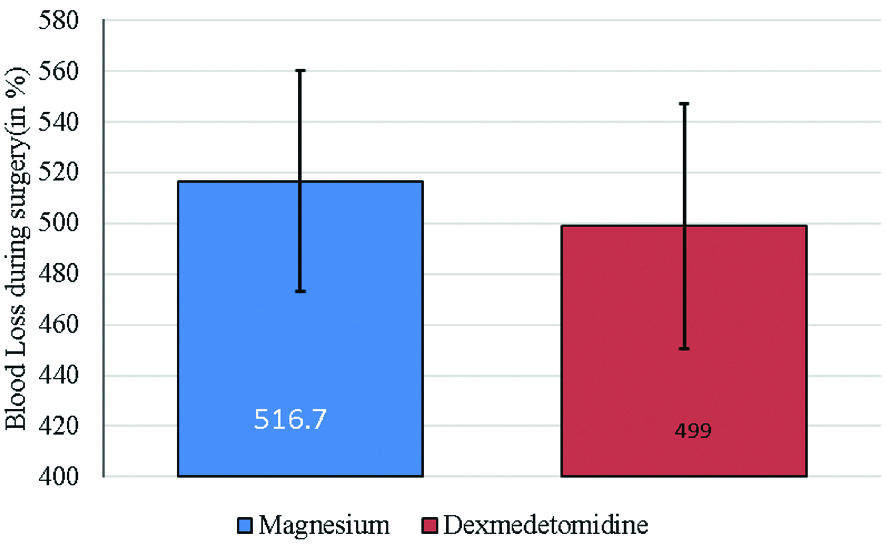
Patients in magnesium sulphate group had a mean blood loss of 516±8.026 mL compared to 499±5.34 mL mean blood loss in Dexmedetomidine group. This was statistically not significant (p>0.05) [Table/Fig-3].
Mean amount of blood loss during surgery among study subjects.
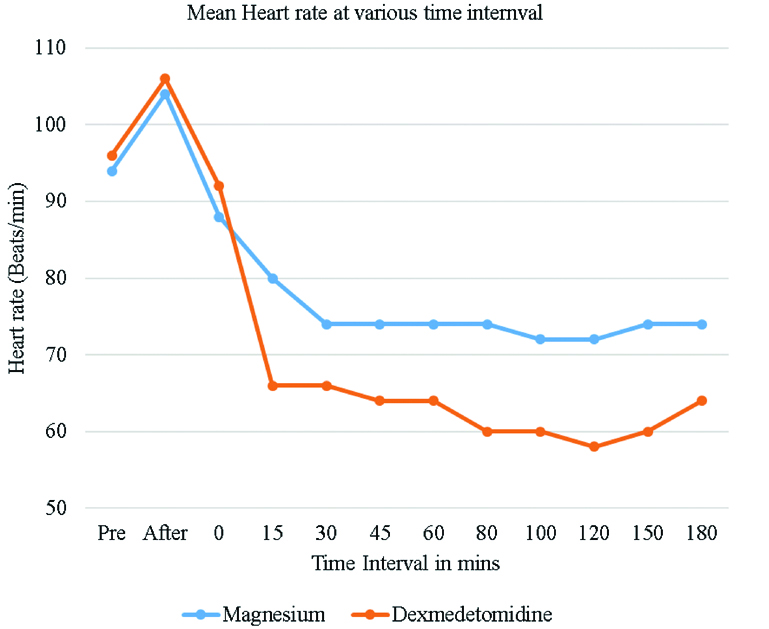
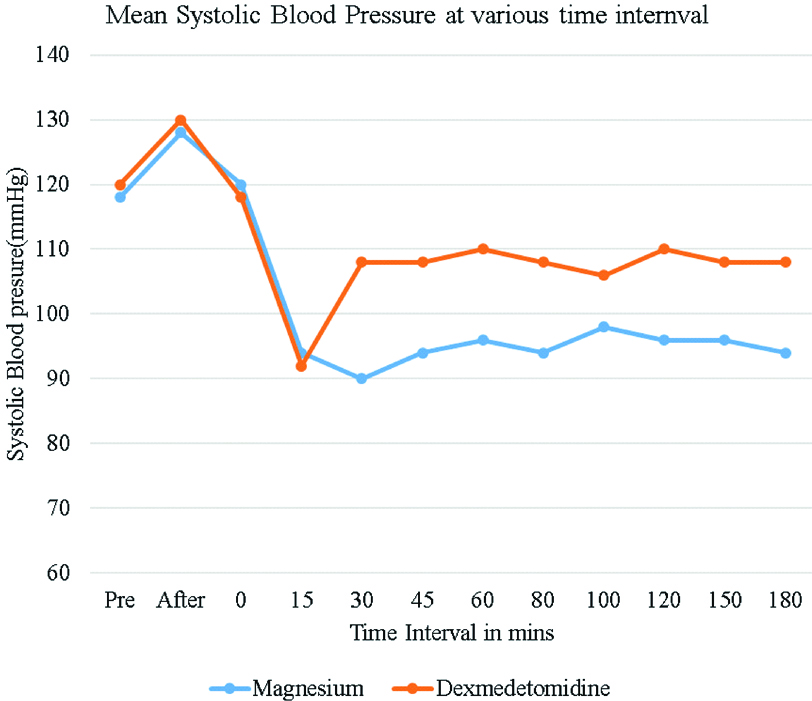
Heart rate variation between the magnesium sulphate group and Dexmedetomidine group were statistically significant (p<0.05) 15 minutes after starting the drug infusions and remained this way throughout the course of the surgery. The mean heart rate was lower in the Dexmedetomidine group as compared to magnesium sulphate group but none of the patients required any intervention. The lowest heart rate in Dexmedetomidine group was 58/min Vs 72/min in magnesium sulphate group [Table/Fig-4]. SBP (Systolic Blood Pressure) and DBP (Diastolic Blood Pressure) were significantly lower in magnesium sulphate group when compared to Dexmedetomidine group throughout surgery after starting the drug infusions and was statistically significant (p<0.05). The lowest median SBP was 90 mmHg in magnesium sulphate group at 30 minutes, and the lowest SBP in Dexmedetomidine group was 92 mmHg after 15 minutes [Table/Fig-5].
Mean heart rate levels among study subjects at various times intervals.
Mean systolic blood pressure levels among study subjects at various times intervals.
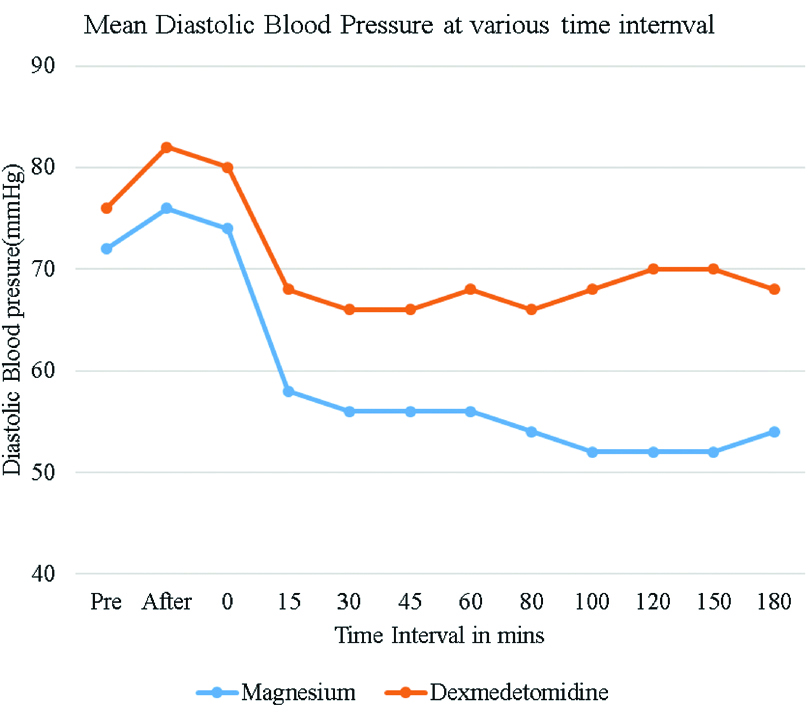
The lowest median DBP was 52 mmHg at 120 minutes in magnesium sulphate group Vs 66 mmHg at 80 minutes in Dexmedetomidine group [Table/Fig-6].
Mean diastolic blood pressure levels among study subjects at various times intervals.
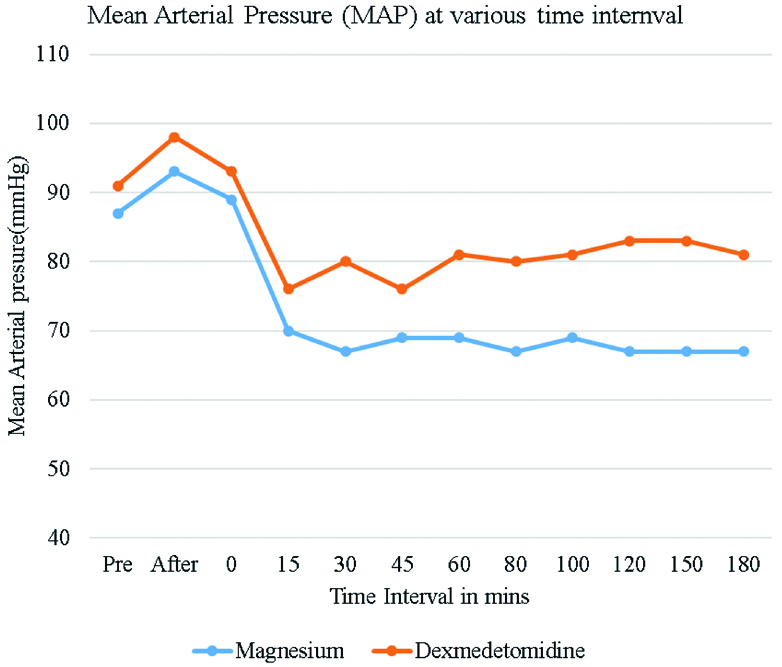
MAP was lower in magnesium sulphate group compared to Dexmedetomidine group starting 15 minutes after infusion of drugs and was statistically significant (p <0.05). The lowest MAP was 67 mmHg in magnesium sulphate group Vs 76 mmHg in Dexmedetomidine group [Table/Fig-7]. Patients in magnesium sulphate group required more ephedrine boluses compared to Dexmedetomidine group (11 doses Vs 4 doses) (p<0.05).
Mean arterial blood pressure levels among study subjects at various times intervals.
Discussion
As the magnitude and complexity of spinal surgery keeps escalating, surgeons and anaesthesiologists should anticipate potential for greater blood loss during procedures. Blood loss during the course of the surgery could result in severe patient complications during and after surgery, it also makes the surgical visualization in blood filled field difficult for the surgeons. The benefits of controlled hypotension during spine surgery include reduction in blood loss as well as reduction in need for blood transfusion. It also improves operating conditions by creating bloodless field. In this prospective randomised study magnesium sulphate and Dexmedetomidine were used to compare the amount of blood loss and haemodynamic parameters during lumbar spinal fusion surgeries in adults.
In present study, the mean blood loss in magnesium sulphate group was 516 ml±8.029 mL and in Dexmedetomidine group it was 499 mL±5.34 mL which is not statistically significant. Ghodraty MR et al., compared magnesium and remifentanil for controlled hypotension during lumbar spinal surgeries and concluded that Remifentanil and Magnesium sulphate have a similar hypotensive effect and comparable amount of blood loss without any significant adverse effects [18]. Nazir O et al., conducted a prospective randomized comparative study using Dexmedetomidine and Esmolol for lumbar spine fusion surgeries [19]. Both the infusions were titrated intraoperatively to maintain MAP between 60-65 mmHg. Total intraoperative blood loss in the esmolol and Dexmedetomidine groups were 308.3±47.5 ml and 277.8±8.9 mL respectively; however it was not statistically significant (p >0.05). Jamaliya RH et al., compared Dexmedetomidine (DEX) and Nitroglycerine (NTG) for posterior spinal fixation surgery [20]. Patients were randomly assigned to receive either DEX 1 μg/kg bolus followed by 0.2-0.7 μg/kg/h infusion or NTG 3-5 μg/kg/min infusion to maintain MAP between 65 and 70 mmHg. The blood loss was significantly lesser in the DEX group (422.11±149.34 mL) than the NTG group (564.51±160.88 mL) (p=0.01).
In our study the median heart rate was 80/min in magnesium sulphate group and 66/min in Dexmedetomidine (DEX) group. This shows that heart rate slowed considerably within 15 minutes of starting the infusion in DEX group. Subsequently, the heart rate remained lower in DEX group compared to magnesium sulphate group till the end of surgery, but the heart rate never dropped below 60/min. Our results are comparable with the study conducted by Akkaya A et al., [21]. They compared the effect of magnesium sulphate and Dexmedetomidine during endoscopic sinus surgery. They observed that heart rate was significantly lower in Dexmedetomidine group compared to Magnesium sulphate group (p<0.05).
SBP, DBP and MAP were significantly lower in magnesium sulphate group compared to Dex group. The lowest median SBP was 90 mmHg at 30 minutes in magnesium sulphate group and 92 mmHg at 15 minutes in Dexmedetomidine group. Similarly, DBP were significantly lower in magnesium sulphate group compared to Dexmedetomidine group. The lowest median DBP was 52 mmHg at 120 minutes in magnesium sulphate group Vs 66 mmHg at 80 minutes in Dexmedetomidine group. The lowest MAP in magnesium sulphate group was 67 mmHg Vs 76 mmHg in group Dexmedetomidine. 11 patients in magnesium sulphate group and 4 patients in Dexmedetomidine group required single boluses of Ephedrine to maintain MAP more than 60 mm of Hg.
A similar study was conducted by Bayram A et al., in functional endoscopic sinus surgery using dexmedetomedine at 1 mcg/kg loading dose, followed by 0.5-1 mcg/kg/hr infusion and magnesium sulphate with a loading dose 40 mg/kg, followed by 10-15 mg/kg/hr infusion [17]. This study showed that Dexmedetomidine group had lower HR, SBP, DBP and MAP when compared to magnesium sulphate group. The dosage of both the drugs are higher in this study compared to our study specifically Dexmedetomidine infusion. That may be the reason for the Dexmedetomidine group having lower blood pressures in their study. Another prospective, randomized, double blinded and placebo controlled study comparing Dexmedetomidine and magnesium sulphate on propofol consumption, haemodynamics and postoperative recovery in spine surgery was conducted by Srivatsava VK et al., [22]. They used dexmedetomedine at 1mcg/kg loading dose followed by 0.5 mcg/kg/hr infusion throughout the surgery and magnesium sulphate with a loading dose 50mg/kg followed by 15 mg/kg/hr infusion throughout the surgery. They found that haemodynamic parameters HR, SBP, DBP and MAP were comparatively lower in Dexmedetomidine group compared to magnesium sulphate group.
These results were different from our study, where SBP, DBP and MAP were lower in magnesium group. The dose of Dexmedetomidine we used is lower compared to the dose they have used (0.6 mcg/kg/hr Vs 0.4 mcg/kg/hr) which may also contributed to the difference in results. We also used fixed infusion rates and did not titrate the infusions according to MAP like other studies which might have contributed to the results.
All our patients were monitored postoperatively for 40-50 minutes in the recovery room for the same haemodynamic parameters. Though none of these parameters came back to the pre-anaesthetic level, there were no drop in heart rate and blood pressure compared to the intra operative period. The patients were later transferred to the orthopaedic ward after observation in PACU (Postanaesthesia Care Unit). None of the patients had any untoward haemodynamic effects in the ward during the first 24 hours.
There are some limitations in our study. We included patients of only ASA PS 1 and 2, the hypotensive effect of these two drugs on higher ASA grade patients were not known. Obese patients were also excluded in our study. It is easier to position lean patients in prone position than the obese without raising the epidural venous pressure. The Caloric method of blood loss assessment may have been more accurate than weighing method. BIS monitoring would have been a better choice than gas monitoring for maintaining the conscious level of the patient.
Conclusion
There were no statistically significant difference in the amount of blood loss in the magnesium sulphate and Dexmedetomidine groups, but intravenous Dexmedetomidine produced a better haemodynamic stability compared to magnesium sulphate group. Magnesium sulphate group had more episodes of hypotension with patients requiring more ephedrine boluses.
[1]. Moller H, Hedlund R, Instrumented and non-instrumented posterolateral fusion in adult spondylolisthesis-a prospective randomized study: part 2Spine 2000 25:1716-21.10.1097/00007632-200007010-0001710870149 [Google Scholar] [CrossRef] [PubMed]
[2]. Ani N, Keppler L, Biscup RS, Steffee AD, Reduction of high-grade slips (grades III-V) with VSP instrumentation. Report of a series of 41 casesSpine 1991 16:S302-10.10.1097/00007632-199106001-000251862430 [Google Scholar] [CrossRef] [PubMed]
[3]. Boachie-Adjei O, Do T, Rawlins BA, Partial lumbosacral kyphosis reduction, decompression, and posterior lumbosacral transfixation in high-grade isthmic spondylolisthesis: clinical and radiographic results in six patientsSpine 2002 27:E161-68.10.1097/00007632-200203150-0001911884921 [Google Scholar] [CrossRef] [PubMed]
[4]. Chang KW, McAfee PC, Degenerative spondylolisthesis and degenerative scoliosis treated with a combination segmental rod-plate and transpedicular screw instrumentation system: a preliminary reportJournal of Spinal Disorders 1988 1:247-56. [Google Scholar]
[5]. Seyhan TO, Tugrul M, Sungur MO, Kayacan S, Telci L, Pembeci K, Effects of three different dose regimens of magnesium on propofolrequirements, haemodynamic variables and postoperative pain relief in gynaecological surgeryBr J Anaesth 2006 96(2):247-52.10.1093/bja/aei29116311277 [Google Scholar] [CrossRef] [PubMed]
[6]. Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP, Transfusion medicine: First of two parts-Blood transfusionN Engl J Med 1999 340:438-47.10.1056/NEJM1999021134006069971869 [Google Scholar] [CrossRef] [PubMed]
[7]. Tobias JD, Strategies for minimizing blood loss in orthopedic surgerySemin Hematol 2004 41(suppl 1):145-56.10.1053/j.seminhematol.2003.11.02514872436 [Google Scholar] [CrossRef] [PubMed]
[8]. Degoute CS, Controlled hypotension: a guide to drug choiceDrugs 2007 67(7):1053-76.10.2165/00003495-200767070-0000717488147 [Google Scholar] [CrossRef] [PubMed]
[9]. Clarke RJ, Glasgow NG, Johnson JW, Mechanistic and structural determinants of NMDA receptor voltage-dependent gating and slow mg2+unblockJ Neurosci 2013 33(9):4140-50.10.1523/JNEUROSCI.3712-12.201323447622 [Google Scholar] [CrossRef] [PubMed]
[10]. Na HS, Lee JH, Hwang JY, Ryu JH, Han SH, Jeon YT, Effects of magnesium sulphate on intraoperative neuromuscular blocking agent requirements and postoperative analgesia in children with cerebral palsyBr J Anaesth 2010 104(3):344-50.10.1093/bja/aep37920042475 [Google Scholar] [CrossRef] [PubMed]
[11]. Wu L, Huang X, Sun L, The efficacy of n-methyl-d-aspartate receptor antagonists on improving the postoperative pain intensity and satisfaction after remifentanil- based anaesthesia in adults: A meta-analysisJ Clin Anaesth 2015 27(4):311-24. 10.1016/j.jclinane.2015.03.02025824051 [Google Scholar] [CrossRef] [PubMed]
[12]. Gertler R, Brown HC, Mitchell DH, Silvius EN, Dexmedetomidine: a novel sedative-analgesic agentProceedings (Baylor University Medical Center) 2001 14(1):13-21.10.1080/08998280.2001.1192772516369581 [Google Scholar] [CrossRef] [PubMed]
[13]. Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, The effect of Dexmedetomidine on perioperative hemodynamicsin patients undergoing craniotomyAnaesth Analg 2008 107(4):1340-47.10.1213/ane.0b013e318180429818806050 [Google Scholar] [CrossRef] [PubMed]
[14]. Guo T-Z, Jiang J-Y, Buttermann AE, Maze M, Dexmedetomidine injection into the locus ceruleus produces antinociceptionThe Journal of the American Society of Anaesthesiologists 1996 84:873-81.10.1097/00000542-199604000-000158638842 [Google Scholar] [CrossRef] [PubMed]
[15]. Guven DG, Demiraran Y, Sezen G, Kepek O, Iskender A, Evaluation of outcomes in patients given Dexmedetomidine in functional endoscopic sinus surgeryAnn Otol Rhinol Laryngol 2011 120(9):586-92.10.1177/00034894111200090622032072 [Google Scholar] [CrossRef] [PubMed]
[16]. Elsharnouby NM, Elsharnouby MM, Magnesium sulfate as a technique of hypotensive anaesthesiaBr J Anaesth 2006 96(6):727-31.10.1093/bja/ael08516670112 [Google Scholar] [CrossRef] [PubMed]
[17]. Bayram A, Ülgey A, Günes I, Ketenci I, Çapar A, Esmaoglu A, Comparison between magnesium sulfate and Dexmedetomidine in controlled hypotension during functional endoscopic sinus surgeryRevistabrasileira de Anestesiologia 2015 65:61-67.10.1016/j.bjane.2014.04.003 [Google Scholar] [CrossRef]
[18]. Ghodraty MR, Homaee MM, Farazmehr K, Nikzad-Jamnani AR, Soleymani-Dodaran M, Pournajafian AR, Comparative induction of controlled circulation by magnesium and remifentanil in spine surgeryWorld J Orthop 2014 5(1):51-56.10.5312/wjo.v5.i1.5124649414 [Google Scholar] [CrossRef] [PubMed]
[19]. Nazir O, Wani MA, Ali N, Sharma T, Khatuja A, Misra R, Dexmedetomidine and Esmolol as Agents to Induce Hypotension in Lumbar Spine SurgeryTrauma Mon 2016 21(3):e2207810.5812/traumamon.2207827921016 [Google Scholar] [CrossRef] [PubMed]
[20]. Jamaliya RH, Chinnachamy R, Maliwad J, Deshmukh VP, Shah BJ, Chadha IA, The efficacy and hemodynamic response to Dexmedetomidine as a hypotensive agent in posterior fixation surgery following traumatic spine injuryJournal of Anaesthesiology Clinical Pharmacology 2014 30:20310.4103/0970-9185.13002124803758 [Google Scholar] [CrossRef] [PubMed]
[21]. Akkaya A, Tekelioglu UY, Demirhan A, Bilgi M, Yildiz I, Apuhan T, Comparison of the effects of magnesium sulphate and Dexmedetomidine on surgical vision quality in endoscopic sinus surgery: randomized clinical studyRevistabrasileira de Anestesiologia 2014 64:406-12.10.1016/j.bjane.2014.01.008 [Google Scholar] [CrossRef]
[22]. Srivastava VK, Mishra A, Agrawal S, Kumar S, Sharma S, Kumar R, Comparative evaluation of Dexmedetomidine and magnesium sulphate on propofol consumption, haemodynamics and postoperative recovery in spine surgery: a prospective, randomized, placebo controlled, double-blind studyAdvanced Pharmaceutical Bulletin 2016 6:7510.15171/apb.2016.01227123421 [Google Scholar] [CrossRef] [PubMed]