Evaluation and Comparison of Blood Parameters in Diabetic Patients with and without Peripheral Neuropathy
KP Ranjith1, Bhanuchandar Potu2, M Anju3, Saleena Ummer Velladath4, Manjunatha Hande5
1 Postgraduate Student, Department of Medical Laboratory Technology, SOAHS, MAHE, Manipal, Karnataka, India.
2 Postgraduate Student, Department of Medical Laboratory Technology, SOAHS, MAHE, Manipal, Karnataka, India.
3 Assistant Professor, Department of Medical Laboratory Technology, SOAHS, MAHE, Manipal, Karnataka, India.
4 Associate Professor and Head, Department of Medical Laboratory Technology, SOAHS, MAHE, Manipal, Karnataka, India.
5 Professor and Head, Department of Medicine, KMC, Manipal, Karnataka, India.
NAME, ADDRESS, E-MAIL ID OF THE CORRESPONDING AUTHOR: Dr. Saleena Ummer Velladath, Associate Professor and Head, Department of Medical Laboratory Technology, SOAHS, MAHE, Manipal, Karnataka, India.
E-mail: saleena.ummer@maniapl.edu
Introduction
Diabetic Peripheral Neuropathy (DPN) is the most prevalent and troublesome microvascular complication (50%) of Diabetes Mellitus (DM). The factors involved in the pathogenesis of diabetic neuropathy have not been understood completely. Studies have shown that neuronal dysfunction and inflammation play important roles in the development of DPN.
Aim
To evaluate and compare the inflammatory marker Neutrophil to Lymphocyte Ratio (NLR) and serum markers calcium, urea, and uric acid in diabetic patients with and without peripheral neuropathy.
Materials and Methods
We prospectively studied 118 diabetic patients who visited Kasturba Hospital, Manipal, Karnataka, India during December 2016- June 2017. After obtaining the ethical approval (IEC 791/2016), the patients were grouped into those with DPN (n=55) and without DPN (n=63). NLR and serum markers calcium, urea, and uric acid were estimated.
Results
The data were analysed using Student’s t-test and Mann-Whitney U test and revealed a significantly high uric acid (p<0.001) and low calcium levels (p<0.001) in DPN patients compared to controls. The NLR was significantly high in DPN group {2.62 (2.3-3.1)} compared to that {1.9 (1.6-2.4)} in non-DPN group (p<0.001).
Conclusion
Monitoring the simple biomarkers routinely in diabetic patients may give an early signal to prevent DPN and thus better management of the patients.
Biomarkers, Calcium, Diabetic peripheral neuropathy, Neutrophil lymphocyte ratio, Uric acid
Introduction
Diabetes mellitus is a universal health problem in the 21st century. It is a non communicable metabolic disorder of multiple aetiology characterised by chronic hyperglycaemia. The incidence of diabetes is increasing to epidemic proportions throughout the world and is considered a major risk to human health. According to the International Diabetes Federation, in 2015, 415 million people worldwide suffered from diabetes, and this number is expected to reach up to 642 million by 2040. In India, more than 62 million individuals currently have diabetes. This figure is expected to rise to more than 123.5 million by 2040 [1]. The most frequently occurring complication in diabetes is DPN or Distal Symmetrical Polyneuropathy (DSP). DPN affects up to 50% of the population with diabetes [2]. Out of this 50%, 16% to 33% of patients manifest neuropathic pain associated with diabetes due to nerve injury, which affects their quality of life [3].
The pathogenesis of DPN is complex and still needs to be explored. Studies have shown that inflammatory processes also play essential roles in the development of DPN. As an inflammatory marker, NLR has a close relationship with many morbid conditions. NLR can reveal the functional status of the immune system in the process of chronic inflammation. In addition, NLR is fairly stable and not easily influenced by physiological, pathological, and physical factors. Thus, NLR may be a good indicator for DPN.
Irregular neuronal calcium balance is involved in numerous diseases of the nervous system. The pathophysiology of two common disorders of the peripheral neuronal system, namely neuropathic pain and diabetic polyneuropathy, has been correlated with calcium channel pathways and functions [4]. Abnormal calcium signalling in diabetes has pathologic significance; elevated calcium influx and cytosolic calcium release have been implicated in neurodegenerative disorders, including diabetes [5]. Elevated serum uric acid may also be associated with increased risk of DPN and may be involved in the aetiology of DPN [6]. Patients with elevated urea levels have been reported to suffer from DPN [7].
In routine clinical practice, diabetic neuropathy is diagnosed using neurological tests. A review of various studies reveals the vital roles of blood biomarkers in the pathogenesis of diabetic neuropathy [4-7]. However, there is a dearth of studies showing the significance of these biomarkers in the screening, diagnosis, and prognosis of DPN. Hence, efforts to identify effective biomarkers for screening and monitoring the prognosis of DPN patients are of great clinical importance. This study aimed to assess and compare the inflammatory marker NLR and the serum markers calcium, urea, and uric acid in diabetic patients with and without peripheral neuropathy to discover whether there is any significant difference in these biomarker values between the groups.
Materials and Methods
We conducted a prospective study on diabetic patients attending the Diabetic Clinic at Kasturba Hospital, Manipal after obtaining the informed consent of patients and the approval of the IEC of Kasturba Hospital, MAHE, Manipal, Karnataka, India (IEC No. 791/2016) for a prospective observational study. All patients attending the diabetic clinic of the tertiary care hospital at Udupi, Karnataka, aged between 30 and 75 years diagnosed with DM were included in the study. Pregnant women and patients with other neurological and thyroid disorders were excluded from the study to avoid cofounding variables. The patients were selected from December 2016 until June 2017 based on the eligibility criteria. DPN was confirmed based on clinical data by the diabetologist.
Sample Size
By taking the standard deviation of 20.32, precision 11 based on previous studies and level of significance as 5%, the calculated total sample size was 55 each of DPN cases and controls for a significant result [5]. A total of 118 diabetic patients were included in the study, out of which 55 patients were with DPN (35 male and 20 female) and 63 patients with DM without neuropathy (34 male and 29 female).
Estimation of Biomarkers
Blood samples were collected from each patient for both serum markers and cell counts (with EDTA). Differential leukocyte count was performed in a Coulter cell counter. Serum was separated and refrigerated at -20°C until analysis. The biomarker levels in each serum sample were estimated using commercial reagent kits. For serum calcium, we used a modified Arsenazo III method (Agappe Diagnostics). In neutral medium, Arsenazo III {2,7-(bis (2-arso phenylazo))-1,8 dihydroxynaphthalene-3,6-disulphonic acid} forms a blue complex with calcium, and the colour intensity produced is measured spectrophotometrically which is directly proportional to the calcium concentration [8,9].
Serum uric acid was estimated using the uricase N-ethyl-N-(3-sulfopropyl)-3-methylaniline sodium salt (TOPS) method (Agappe Diagnostics). Uricase transforms uric acid into allantoin with the formation of hydrogen peroxide in the presence of Peroxidase (POD). It reacts with Ethyl Sulfopropyl Toluidine (ESTP) and 4-aminophenazone to produce a coloured complex with a colour intensity directly proportional to the uric acid concentration in the sample [10].
For serum urea, the modified Berthelot method (Agappe Diagnostics) was used, in which urea is converted to ammonia and carbon dioxide using urease enzyme. The ammonia formed is then transformed into blue dicarboxy endo phenol, the colour intensity of which is directly proportional to the amount of urea in the blood [11,12].
The values of Fasting Blood Glucose (FBG), Post Prandial Blood Glucose (PPBG), HbA1C, complete blood count and ESR were collected prospectively from the routine clinical data.
Statistical Analysis
Data were entered and analysed using Statistical Package for the Social Sciences (SPSS) version 20.0. The continuous variables were summarised as a mean and standard deviation or median and interquartile range based on the data distribution. The comparison of variables that followed a normal distribution was made using an Independent t-test, and comparison of those that did not follow a normal distribution was done using the Mann-Whitney U test. Categorical variables were summarised as percentages, and their comparison was made using a chi-square test. A probability of 0.05 or less was considered significant. The cut-off values for each biomarker were determined using Receiver Operating Characteristic (ROC) analysis.
Results
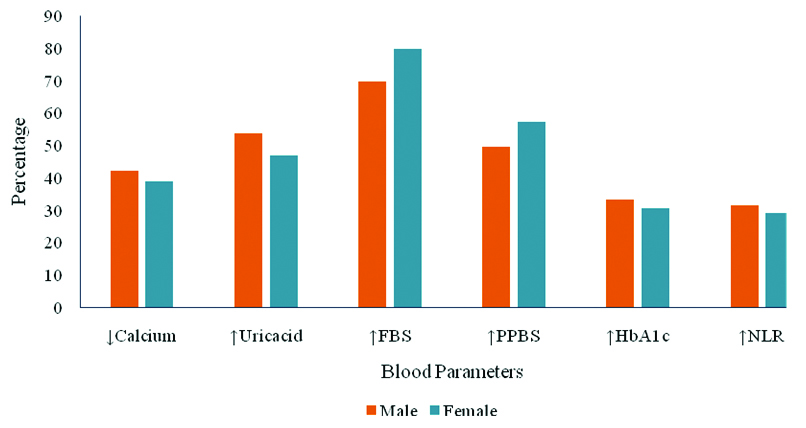
A total of 118 diabetic patients were included in the study, of which 55 suffered from DPN. The median age was 57 years (range: 43-67 years) in the DPN group and 52 years (range: 38-65 years) in patients without DPN (p=0.013). The median duration of diabetes was 10 years (range: 6-14) and 7 years (range: 3-10) in patients with DPN and without DPN, respectively (p=0.008). The comparison of demographic data showed a significant difference only in terms of age and duration of diabetes [Table/Fig-1]. There was no significant difference in blood parametes between males and females [Table/Fig-2].
Comparison of demographic features.
| Variables | Diabetic patients with DPN (n=55) | Diabetic patients without DPN (n=63) | p-value |
|---|
| Age in years(Median and IQR) | 57 (43-67) | 52 (38-65) | 0.013* |
| Duration in years(Median and IQR) | 10 (6-14) | 7 (3-10) | 0.008* |
| Weight (kg)(Mean±SD) | 65.21±12.35 | 65.24±11.73 | 0.992 |
| Gender (M/F) | (1.75) 35/20 | (1.21) 34/29 | 0.084 |
| BMI (kg/m2) | 25.11±11.26 | 24.84±17.73 | 0.261 |
*Significant difference between the groups, PN: Peripheral neuropathy; BMI: Body mass index
Genderwise comparison of blood parameters.
Significance of Biochemical and Haematological Parameters in DPN
When the laboratory data were compared, serum uric acid (7.93±1.46 mg/dL) and neutrophils (91.4±16.7%) were significantly higher in the DPN group (for both p<0.001). Mean serum calcium was lower (7.59±0.62 mg/dL) in the DPN group than among patients without DPN (10.19±0.67 mg/dL) (p<0.001). The difference in blood urea values between the groups was not significant [Table/Fig-3]. A significantly high count was observed in neutrophils (p<0.001) and total WBC (p=0.009) in DPN group. The NLR in patients with DPN {2.6 (2.1-3.1)} was significantly high when compared to non-DPN group (p<0.001) [Table/Fig-4].
Comparison of biochemical parameters in diabetic patients with and without DPN.
| Parameters | With DPN (n=55) | Without DPN (n=63) | p-value |
|---|
| Calcium (mg/dL) | 7.59±0.62 | 10.19±0.67 | <0.001* |
| Uric acid (mg/dL) | 7.93±1.46 | 4.71±1.05 | <0.001* |
| Urea (mg/dL) | 23 (19-31) | 23 (20-29) | 0.990 |
| FBG (mg/dL) | 184.46±72.86 | 163.25±46.27 | 0.101 |
| PPBG (mg/dL) | 239.67±77.62 | 220.55±69.75 | 0.289 |
| GlyHb (%) | 9.07 (7.10-11.49) | 7.83 (6.90-9.70) | 0.177 |
*Significant difference between the groups, FBG: Fasting blood glucose; PPBG: Post prandial blood glucose; GlyHb: GlycatedHb (HbA1C); AST: Aspartate transaminase; ALT: Alanine transaminase; ALP: Alkaline phosphatase
Comparison of haematological parameters in diabetic patients with and without DPN.
| Parameters | With DPN (n=55) | Without DPN (n=63) | p-value |
|---|
| Hb | 12.13±2.18 | 12.46±2.10 | 0.599 |
| RBC count (×106/μL) | 5.4±2.1 | 5.6±3.2 | 0.133 |
| PCV (%) | 46±5.2 | 47±4.8 | 0.372 |
| Platelet (×103/μL) | 173±23.2 | 173±29.4 | 0.724 |
| ESR | 30(9-47) | 31 (13-47) | 0.693 |
| WBC count (×103/μL) | 9.97±3.1 | 7.56±2.78 | 0.009* |
| Neutrophils (%) | 91.4±16.7 | 71.3±16.8 | <0.001* |
| Lymphocyte (%) | 35.2±12.8 | 36.7±13.5 | 0.537 |
| NLR | 2.6 (2.1-3.1) | 1.9 (1.6-2.4) | <0.001* |
*Significant difference between the groups, Hb: Haemoglobin; RBC: Red blood cell; PCV: Packed cell volume; ESR: Erythrocyte sedimentation rate; WBC: White blood cell; NLR: Neutrophil lymphocyte ratio
Predictive Characteristics of Biomarkers
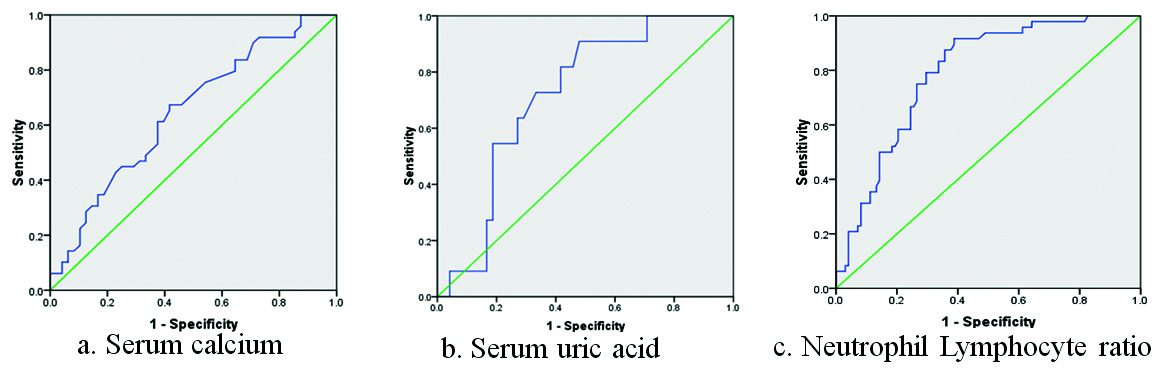
The cut-off values obtained through ROC analysis for serum calcium, Serum uric acid and NLR were 7.91 mg/dL, 6.67 mg/dL and 2.26 respectively [Table/Fig-5]. The sensitivity and specificity for serum calcium were 84.2% and 51.4% respectively with an Area Under the Curve (AUC) of 0.67 (95% CI, 0.62-0.73) [Table/Fig-6a]. The cut-off value obtained for serum uric acid was at sensitivity 86.3% and specificity 52.7% with AUC of 0.71 (95% CI, 0.65-0.74) [Table/Fig-6b]. The AUC for NLR was 0.73 (95% CI, 0.65-0.77) with 87.8% sensitivity and 57.3% specificity [Table/Fig-6c].
Predictive characteristics of biomarkers.
| Biomarker | Opt. Cut-off value | Sensitivity | Specificity | AUC | 95% CI of AUC |
|---|
| Serum calcium (mg/dL) | 7.91 | 84.2% | 51.4% | 0.67 | 0.62-0.73 |
| Serum Uric acid (mg/dL) | 6.67 | 86.3% | 52.7% | 0.71 | 0.65-0.74 |
| NLR | 2.26 | 87.8% | 57.3% | 0.73 | 0.65-0.77 |
NLR: Neutrophil lymphocyte ratio; CI: Confidence interval; AUC: Area under the curve
Receiver operating characteristic (ROC) analysis for the biomarkers to determine the cut-off values.
Discussion
Diabetic peripheral neuropathy is the most common complication of DM having high morbidity and mortality. Early diagnosis and protective measures can reduce this burden to a great extent. This study was an attempt to find out the significance of few blood biomarkers in patients with DM which may be useful for the screening of DPN. We observed a significantly increased level of serum uric acid and low serum calcium in patients with DPN compared to non-DPN patients. A previous study was done by Hough F et al., which reported calcium dysregulation in both painful and degenerative diabetic neuropathies that correlate with our finding of low serum calcium in DPN cases compared to controls [4].
In present study, the proportion of hyperglycaemia was slightly more in females when studied FBS, while increased PPBS, and HbA1c levels were seen more in males. The proportion of hypocalcaemia, hyperuricaemia, and NLR were slightly more in males. However, there was no significant difference in these parameters between the gender. Hence, we hope the number of males and females included in the study sample may not be affecting the outcome of the study significantly.
The data from one meta-analysis suggest that high uric acid is associated with high severity of DPN and is involved in the pathophysiology. Since, uric acid level in serum is an adjustable factor, uric acid lowering treatments may have some impact in preventing DPN. However, there is no report on the effects of uric acid lowering therapies in the prevention and curing of DPN [13], which may be a quest for future research. Hovind P et al., studied patients with type 1 diabetes and showed that uric acid level was independently correlated with the risk for the later progress of diabetic neuropathy [14]. The calcium “set point” hypothesis recommend that modest alteration in cytosolic calcium over lengthen periods may be harmful to the cell. It was noted that neuronal resting intracellular Ca2+ abnormalities are increased progressively with the duration of diabetes [15].
Diabetic neuropathy is associated with a number of cellular and systemic processes that manifest as inflammatory dysfunction. We observed significantly high NLR being a marker of inflammation in DPN patients. Diabetic neuropathy is associated with a number of cellular and systemic processes that manifest as markers of endothelial and inflammatory dysfunction. As the low-grade inflammation is a contributing factor of diabetic neuropathy, the current research on DPN therapy is targeting inflammation that may alleviate diabetic neuropathy [16-18].
Limitation
Though we could found high NLR, high uric acid, and low calcium are associated with DPN, we need to confirm these findings in larger cohorts. This study may not be sufficient to say the association of these markers with non-diabetic neuropathy, as we have not included patients with other neuropathies.
Conclusion
As the duration of DM and age of a patient are positively associated with the risk of DPN, associated hyperuricaemia and hypocalcaemia with high NLR may be a warning signal for peripheral neuropathy in diabetic patients. Early detection of sensory loss in the lower extremities is essential to prevent the development of neuropathic ulceration and amputation. Control of glucose, blood pressure and lipids together with uric acid and calcium levels may help better in preventing further complications.
*Significant difference between the groups, PN: Peripheral neuropathy; BMI: Body mass index
[1]. Region E, Contribute data to the 7th edition of IDF’s Diabetes AtlasDiabetes Research and Clinical Practice 2015 107:30810.1016/j.diabres.2014.12.003 [Google Scholar] [CrossRef]
[2]. Davies M, Brophy S, Williams R, Taylor A, The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetesDiabetes Care 2006 29(7):1518-22.10.2337/dc05-222816801572 [Google Scholar] [CrossRef] [PubMed]
[3]. Saxena AK, Nath S, Kapoor R, Diabetic peripheral neuropathy: current concepts and future perspectivesJournal of Endocrinology and Diabetes 2015 2(5):01-08. [Google Scholar]
[4]. Fernyhough P, Calcutt NA, Abnormal calcium homeostasis in peripheral neuropathiesCell Calcium 2010 47(2):130-39.10.1016/j.ceca.2009.11.00820034667 [Google Scholar] [CrossRef] [PubMed]
[5]. Kumar PS, Ramakrishna T, Sreekumaran E, Association of diabetic neuropathy and low serum calcium levels in diabetic patientsInternational Journal of Scientific & Engineering Research 2015 6(1):767-69. [Google Scholar]
[6]. Yu S, Chen Y, Hou X, Xu D, Che K, Li C, Serum uric acid levels and diabetic peripheral neuropathy in type 2 diabetes: A systematic review and meta-analysisMolecular Neurobiology 2016 53(2):1045-51.10.1007/s12035-014-9075-025579387 [Google Scholar] [CrossRef] [PubMed]
[7]. Aguiar PCM, Coletta MVD, de Souza JJS, The association of dyslipidemia and peripheral diabetic neuropathy: the influence of ureaDiabetol Metab Syndr 2015 7(Suppl 1):A3010.1186/1758-5996-7-S1-A30PMC4653427 [Google Scholar] [CrossRef] [PubMed]
[8]. Janssen JW, Helbing AR, Arsenazo III: an improvement of the routine calcium determination in serumEuropean Journal of Clinical Chemistry and Clinical Biochemistry: Journal of the Forum of European Clinical Chemistry Societies 1991 29(3):197-201. [Google Scholar]
[9]. Morgan BR, Artiss JD, Zak B, Calcium determination in serum with stable alkaline Arsenazo III and triglyceride clearingClinical Chemistry 1993 39(8):1608-12.10.1093/clinchem/39.8.16088353945 [Google Scholar] [CrossRef] [PubMed]
[10]. Sanders GT, Pasman AJ, Hoek FJ, Determination of uric acid with uricase and peroxidaseClinica Chimica Acta 1980 101(2-3):299-303.10.1016/0009-8981(80)90257-0 [Google Scholar] [CrossRef]
[11]. Fawcett JK, Scott J, A rapid and precise method for the determination of ureaJournal of Clinical Pathology 1960 13(2):156-59.Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC480024/pdf/jclinpath00055-0064.pdf10.1136/jcp.13.2.15613821779 [Google Scholar] [CrossRef] [PubMed]
[12]. Fawcett JK, Scott J, A rapid and precise method for the determination of ureaJournal of Clinical Pathology 1960 13(2):156-59.10.1136/jcp.13.2.15613821779 [Google Scholar] [CrossRef] [PubMed]
[13]. Kiani J, Habibi Z, Tajziehchi A, Moghimbeigi A, Dehghan A, Azizkhani H, Association between serum uric acid level and diabetic peripheral neuropathy: A case control studyCaspian Journal of Internal Medicine 2014 5(1):17 [Google Scholar]
[14]. Hovind P, Rossing P, Tarnow L, Johnson RJ, Parving HH, Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort studyDiabetes 2009 58(7):1668-71.10.2337/db09-001419411615 [Google Scholar] [CrossRef] [PubMed]
[15]. Migdalis IN, Xenos K, Chairopoulos K, Varvarigos N, Leontiades E, Karmaniolas K, Ca2+-Mg2+-ATPase activity and ionized calcium in Type 2 diabetic patients with neuropathyDiabetes Research and Clinical Practice 2000 49(2):113-18.10.1016/S0168-8227(00)00150-9 [Google Scholar] [CrossRef]
[16]. Pop-Busui R, Ang L, Holmes C, Gallagher K, Feldman EL, Inflammation as a therapeutic target for diabetic neuropathiesCurrent Diabetes Reports 2016 16(3):2910.1007/s11892-016-0727-526897744 [Google Scholar] [CrossRef] [PubMed]
[17]. Jin HY, Park TS, Role of inflammatory biomarkers in diabetic peripheral neuropathyJournal of Diabetes Investigation 2017 Dec 26 Available from: https://doi.org/10.1111/jdi.1279410.1111/jdi.1279429277966 [Google Scholar] [CrossRef] [PubMed]
[18]. Gedela S, Rao AA, Medicherla NR, Identification of biomarkers for type 2 diabetes and its complications: a bioinformatic approachInternational Journal of Biomedical Science 2007 3(4):229 [Google Scholar]