The inherited disorders of haemoglobin pose a significant health burden in many countries in the Mediterranean region, the Middle and Far East, Africa and South Asia [1]. Beta thalassaemia is prevalent in many population groups in India including the marginalized populations living in rural and often remote areas. Many of these populations also harbor the sickle cell gene in variable proportions [2]. Both these disorders cause considerable morbidity and mortality and the only way to reduce this health burden is by spreading awareness in these communities, followed by screening for identification of carriers, genetic and marriage counselling and preventing the birth of affected children by prenatal diagnosis [2,3].
There are many different approaches for screening to identify heterozygotes of beta thalassaemia and it is well accepted that the most suitable and accurate way would be to measure the red cell indices on an automated cell counter and at the same time estimate the levels of HbA2, HbF or other Hb variants using the commercially available dedicated HPLC or Capillary Electrophoresis machines. Majority of the beta thalassaemia carriers would have reduced red cell indices (MCV < 80 fl and/or MCH < 27 pg) with elevated HbA2 levels (> 3.5%) and HbS or any other Hb variant would also be identified [4,5].
This ideal approach is possible in well established centres or hospitals where the cost of the test is not a major problem and where facilities are readily available, however the scenario is different in many rural regions and a simple test like the Naked Eye Single Tube Red Cell Osmotic Fragility Test (NESTROFT) has been used for preliminary screening at the primary health care level.
There are inherent problems with NESTROFT like false positive and false negative findings due to various reasons and we have tried to resolve some of these issues. The aim of this study was to modify the conventional NESTROFT solution to increase its stability and evaluate its sensitivity and specificity for detection of β-thalassaemia carriers and HbS carriers.
Materials and Methods
Subjects: The present observational study comprised of total 157 individuals of both sexes. This included individuals and families referred to us for screening and prenatal diagnosis of haemoglobinopathies. An informed consent was taken before blood collection and the study was approved by our Institutional Ethics Committee.
Methods: Screening by NESTROFT was done using both solution 1 and 2 given below.
Two stock solutions of 3.6% phosphate buffered saline were prepared as follows and stored at 40C.
Solution 1
Sodium Chloride (NaCl) - 32.4 g
Disodium Phosphate (Na2HPO4) - 4.91g
Sodium Dihydrogen Phosphate (NaH2PO4). 2H2O - 0.87g
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)- 1.0 g
HPLC grade water to 1 litre (pH 7.4)
(HPLC grade water is water which is free from organic and inorganic compounds and does not have any UV absorbance and is filtered through a 0.22 micron filter to avoid any particulate matter).
Solution 2
Sodium Chloride (NaCl) - 32.4 g
Disodium Phosphate (Na2HPO4) - 4.91g
Sodium Dihydrogen Phosphate (NaH2PO4).2H2O - 0.87g
HPLC grade water to 1 litre (pH 7.4)
The working solutions 1 and 2 (0.36% buffered saline) were prepared freshly just before putting up the test by a 1 in 10 dilution of the stock solution using HPLC grade water. The same stock solutions were used for up to 6 months and the pH measured periodically. The HEPES containing solution was kept in darkness as much as possible to avoid phototoxicity. The procedure followed was as described earlier [6]. A 2.0 mL of 0.36% working solution 1 and solution 2 were taken in corning glass tubes (100 mm X 10 mm). A 20 μL of freshly collected blood in EDTA was added to each tube. The tubes were mixed by inversion and allowed to stand at room temperature for 25-30 minutes. Turbidity was then visually assessed by holding a white paper with a sharp black thin line behind the tube. The results were recorded as positive (+) if the line was not visible, doubtful if it was partially visible (+/-) and negative (-) if it was clearly visible. Both the positive (+) and doubtful (+/-) samples were considered to be positive while calculating the sensitivity and specificity of the 2 reagents as all these samples would be processed further for HPLC analysis in a screening programme.
CBC was done on the Sysmex K 1000 analyser and HPLC analysis was also done in all the subjects on the Variant II Haemoglobin analyser from BioRad laboratories. This was done to differentiate three distinct groups which included 77 normal individuals without beta thalassaemia trait, 68 beta thalassaemia carriers and 12 sickle cell carriers.
Results
The individuals were divided in 3 groups for analysis as shown in [Table/Fig-1]. The normal group included 77 individuals who had MCV>76 fl and MCH> 25pg with Hb A2 levels between 1.6 and 3.2% with normal HbF levels and they did not show the presence of any variant haemoglobin on HPLC. The second group of 68 individuals had MCV<80 fl and MCH < 27 pg with HbA2 > 3.5%. They were grouped as beta thalassaemia traits. However, in this group one individual was a silent carrier and had Hb A2 of 3.2% and 3 individuals had MCV> 80fl and MCH > 27 pg but they were confirmed to be beta thalassaemia carriers by molecular analysis. The third group included 12 individuals who were sickle cell (HbS) carriers.
Results of NESTROFT using two different buffered solutions.
| By HPLC | NESTROFTSolution 1 (With HEPES) | NESTROFTSolution 2 (Without HEPES) |
|---|
| + | +/- | - | + | +/- | - |
|---|
| Normal(n= 77) | 1(1.3%) | 15(19.5%) | 61(79.2%) | 2(2.6%) | 18(23.4%) | 57(74.0%) |
| β-thalassaemia trait(n= 68) | 52(76.5%) | 16(23.5%) | 0(0.0%) | 46(67.6%) | 19(28.0%) | 3(4.40%) |
| HbS trait(n= 12) | 6(50.0%) | 6(50.0%) | 0(0.0%) | 6(50.0%) | 2(16.7%) | 4(33.3%) |
| Total(n =157) | 59(37.5%) | 37(23.5%) | 61(39.0%) | 54(34.4%) | 39(25.0%) | 64(40.8%) |
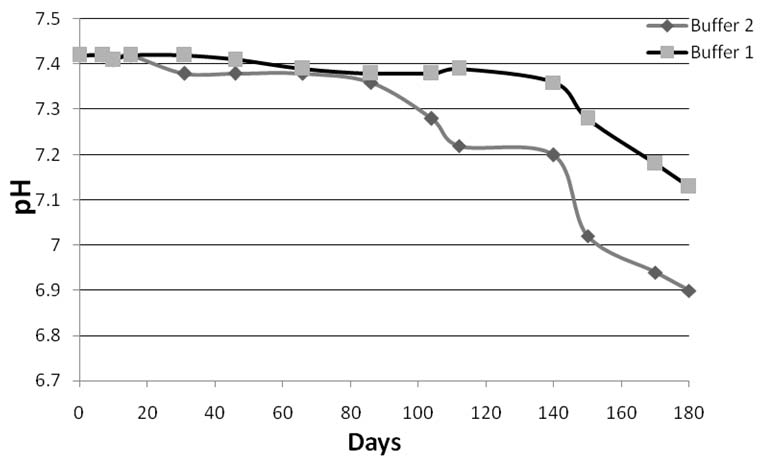
Using solution 1 which contained HEPES, 16 individuals (20.8%) in the normal group were NESTROFT (+) or (+/-), all the 68 beta thalassaemia carriers (100%) were NESTROFT (+) or (+/-) and all the 12 HbS carriers (100%) were also NESTROFT (+) or (+/-). Using solution 2 which did not have HEPES, 3 beta thalassaemia carriers (4.4%) and 4 Hb S carriers (33.3%) were NESTROFT (-). The sensitivity and specificity of NESTROFT using the 2 solutions and the positive and negative predictive values are shown in [Table/Fig-2]. The solution with HEPES (solution 1) had a sensitivity of 100% and specificity of 79.22% with a positive predictive value of 81% and a negative predictive value of 100%. Thus, it was better than the standard solution without HEPES (Solution 2). [Table/Fig-3] shows the change in pH of the 2 stock solutions over a period of 6 months. The NESTROFT buffer which contained HEPES maintained the pH up to 150 days while the pH of the buffer without HEPES started dropping after 90 days.
Sensitivity, specificity, positive and negative predictive values of the two solutions for screening for beta thalassemia carriers.
| Solution1(with Hepes) | Solution 195% Cl | Solution 2(without HEPES) | Solution 295% CI |
|---|
| Sensitivity | 100.0% | 94.7 - 100% | 95.6% | 87.6 - 99.0% |
| Specificity | 79.2% | 68.5 - 87.6% | 74.0% | 62.8 - 83.4% |
| Positive Predictive Value | 81.0% | 70.9 - 88.7% | 76.5% | 66.0 - 85.0% |
| Negative Predictive Value | 100.0% | 93.0 - 99.8% | 95.0% | 86.0 - 99.0% |
Change in pH of the two stock solutions (Buffer 1-with HEPES and Buffer 2-without HEPES) over a period of 6 months.
Discussion
The thalassaemia and sickle cell disorders are widely prevalent in India with the average prevalence of beta thalassaemia carriers in the population being 3-4% and several castes as well as some tribal groups having much higher frequencies ranging from 5 to 17% [2,3]. The carrier rates of HbS also vary from 1 - 35% in different tribal and scheduled caste groups in the country [7,8]. A large majority of the screening programmes for beta thalassaemia have been done in urban areas and many population groups residing in rural areas have not been screened. With about 70% of the population residing in rural regions, screening for carriers of these haemoglobin abnormalities is required in these rural and often remote regions to reduce the national health burden of haemoglobinopathies.
Along with the haemoglobin level, measuring the red cell indices on an electronic cell counter and measuring the different fractions of haemoglobin by HPLC or Capillary Electrophoresis would be the ideal way to identify carriers of beta thalassaemia and other haemoglobin variants [2,4]. However, this is not always feasible. In remote rural areas there are often many problems in the maintenance, calibration and use of equipment like automated haematology counters and HPLC machines. Trained technical staff to operate these equipments is often not available due to frequent change and transfer of staff. Compounded with this is the problem of power shortage and load shedding leading to electricity not being available for extended periods of time. In such situations a simple test like NESTROFT which does not require any equipment or specialized training could be useful as a first line screen for carriers of beta thalassaemia.
NESTROFT has been used in many screening programmes in India and it has been shown to have a high sensitivity but lower specificity. The sensitivity has ranged from 91-100% and the specificity has ranged from 66.6 to 100% with the positive predictive value ranging from 33.6 to 100% and negative predictive value ranging from 83.3 to 100% in different studies. These screening programmes have been done for antenatal women, high-risk communities and siblings and family members of beta thalassaemia major patients [6,9-20]. The present study included individuals and families referred for screening and couples referred for prenatal diagnosis. The CBC and HPLC analysis for HbA2 and HbF estimation and identification of Hb variants in all the cases was done. This was a validation study where comparision of the sensitivity and specificity of the two NESTROFT solutions for picking up beta thalassaemia carriers was done. The modified NESTROFT solution containing HEPES had a higher sensitivity (100%) and specificity (79.22%) compared to the conventional NESTROFT solution which gave a sensitivity of 95.6% and specificity of 74.03%. The buffer with HEPES also had a negative predictive value of 100% compared to 95% in the one without HEPES. False positive results were seen in 20.8% of the 77 individuals grouped as normals using the HEPES buffer and in 26% of individuals using the conventional NESTROFT solution. Thus the modified buffer with HEPES was found to be better than the conventional buffer. The modified NESTROFT solution containing HEPES is a novel solution and has never been used in any earlier studies and therefore comparison of its impact on results as well as its application in practice with other earlier studies was not possible.
NESTROFT is only a preliminary screening test to suspect the presence of beta thalassaemia trait, however, it can also give false positive results in cases with Iron Deficiency Anaemia (IDA) or alpha thalassaemia carriers, both of which are common in India [21,22] and this may be one of the reasons for the higher number of false positive results seen in different studies. In this study, IDA was not ruled out, however, CBC and HPLC was done in all cases where NESTROFT was positive, the beta thalassaemia carriers were differentiated from IDA and alpha thalassaemia carriers at this second stage.
A recent analysis of data from different studies on NESTROFT, also known as the One Tube Osmotic Fragility Test (OTOFT) has suggested that the associated presence of alpha thalassaemia, glucose-6-phosphate dehydrogenase (G6PD) deficiency or Southeast Asian Ovalocytosis (SAO) in beta thalassaemia carriers could reduce the sensitivity of the test for beta thalassaemia heterozygosity to less than 70% [23].
NESTROFT has also been used in many other resource limited countries like Thailand, Iran, Egypt, Pakistan and Bangladesh and the sensitivity and specificity has been similar to the studies reported from India [24-29]. In some of these populations IDA and alpha thalassaemia are also prevalent.
Thus, the problem of false negative results does remain which would miss to pick up some beta thalassaemia carriers.
In the large multi-centre study done by the Indian Council of Medical Research it was found that the main reasons for false negative results in NESTROFT was the quality of water available in different rural areas for preparing the reagent, weighing balances not being properly calibrated leading to inaccurate weighing of chemicals and problems in dilution from the 10% stock buffer solution to the 0.36% working solution. Frequent change of technicians putting up the test also led to a greater number of false negative and false positive results [30].
In this study, an attempt was made to circumvent some of these problems to try and make the test more reliable and user friendly. Several issues were addressed. Firstly, preparing the stock buffer centrally in a well established laboratory and supplying it to rural regions would lead to the same standardized reagent being used by all the centres performing the test. Secondly, supplying HPLC grade water to the centres for diluting the stock solution would resolve the problem of the quality of water used by different centres and thirdly, preparing a 3.6% stock solution rather than a 10% stock solution would mean a single 1:10 dilution to be done which would minimize errors related to the concentration of the working buffer solution. We also modified the stock solution by adding HEPES to it to maintain the pH for a longer duration. When both these solutions were compared, cost wise with each other, it was found that addition of HEPES in the solution does not affect the cost. Additional cost is negligible.
When compared the solution containing HEPES (Solution 1) with the Standard solution without HEPES (Solution 2) over a period of 6 months. Solution 1 with HEPES was able to pick up all the beta thalassaemia heterozygotes as NESTROFT (+) or (+/-) while the solution without HEPES missed 3 beta thalassaemia heterozygotes (4.4%). The 12 sickle cell traits were also picked up by Solution 1 as (+) or (+/-) while solution 2 missed 4 sickle heterozygotes (33.3%). The pH of the stock solution 1 could be maintained for a longer duration.
Limitation
The limitation of the study is the small sample size and performing the test in a well established laboratory in an urban setting. NESTROFT, using this modified solution should first be validated in a rural setting on a much larger sample size along with CBC and HPLC analysis before it is used for screening of carriers of beta thalassaemia and sickle cell disorders.
Conclusion
This study has shown that the modified solution could be prepared and distributed to different centres along with the water to be used for dilution to increase the sensitivity of the test. Using this solution NESTROFT could be used as a first line population screening test in a rural setting after it has been validated on a larger sample size along with CBC and HPLC analysis. Subsequently CBC and HPLC can only be done in NESTROFT positive cases, thus making it cost effective. It could prove to be a useful and economic approach particularly in settings where cost is an issue and facilities are limited.